Calcite, a crystalline form of calcium carbonate, is a mineral with a rhombohedral appearance. It is commonly found in limestone and marble, making it one of the most abundant minerals on Earth. Calcite is known for its stability compared to other forms of calcium carbonate such as aragonite and vaterite.
The study of calcite is crucial due to its broad relevance in various fields. When synthesized, calcite can play a vital role in transforming carbon dioxide into solid carbonate, aiding in long-term carbon storage to combat climate change. Recent research has also shown that defects in calcite, which can be controlled during synthesis, have a significant impact on its properties. These defects can influence its ability to absorb harmful substances like heavy metals, affect its mechanical strength, and enhance catalysts used in industrial processes.
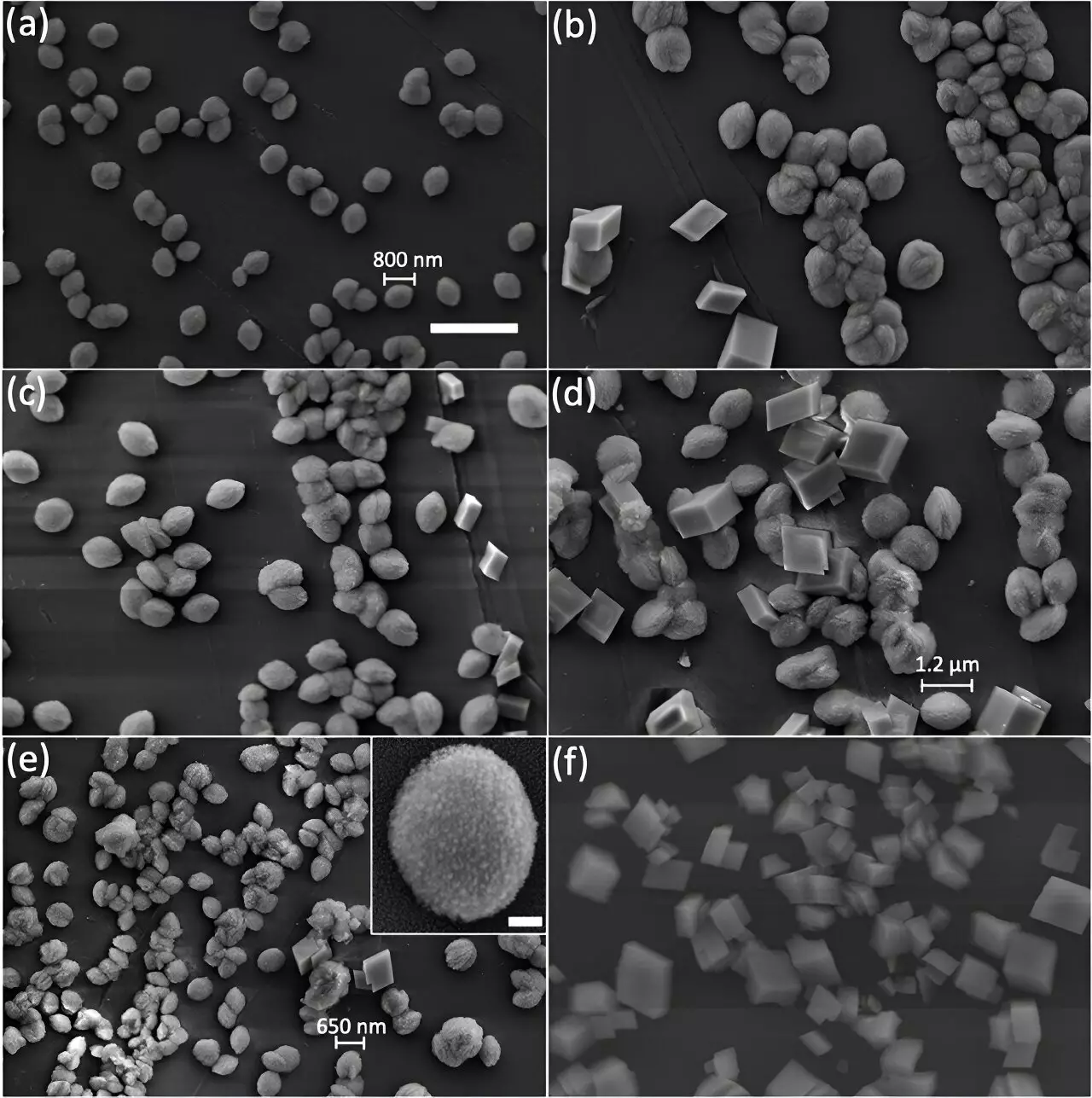
Sang Soo Lee, a geochemist at the U.S. Department of Energy’s Argonne National Laboratory, described calcite as appearing “transparent and shiny” on the surface, resembling a crystal. However, recent studies have shown that the internal structure of calcite can be dramatically altered based on the synthesis approach used. Ana Suzana, along with other researchers at Argonne, discovered that different synthesis methods can lead to various internal structures in calcite particles, ultimately affecting its reactivity.
To understand the impact of synthesis approaches on calcite’s internal structure, Suzana compared particles grown using two different methods. By utilizing scanning electron microscopy (SEM), powder X-ray diffraction, and Bragg Coherent Diffraction Imaging (BCDI) at Argonne’s Advanced Photon Source, researchers were able to visualize the internal nanocrystallinity of calcite. The BCDI technique provided a high-resolution view, enabling the observation of localized features within individual mineral particles.
The study revealed that slow growth of calcite crystals produced particles with expected rhombohedral shapes and orderly internal patterns. However, rapidly grown crystals exhibited a more complex internal structure with countless nanosized crystalline fragments or defects. These defects resembled the granular substructures often seen in vaterite, a less stable form of calcium carbonate.
The presence of nanoscopic defects in calcite’s internal structure can significantly impact its reactivity and functionality. The ability to differentiate between perfect calcite and fragmented calcite is essential, as the latter can have different chemical reactions and properties. Understanding how these defects alter calcite’s behavior can lead to the development of materials with optimized strength, toughness, and catalytic activities.
Identifying internal fragmentation in mineral structures, as demonstrated in this study, can have far-reaching implications for material design and catalysis. By utilizing advanced imaging techniques like BCDI, researchers can gain valuable insights into the structure-property relationships of minerals like calcite. The results of this research open up new possibilities for controlling and harnessing the reactivity of calcite and other mineral structures in various applications.


Leave a Reply