The process of converting methane into methanol is currently energy and resource-intensive, relying heavily on rare and expensive transition or noble metals. While catalyst systems for direct oxidation of methane to methanol have been developed in the past decade, most of them have been using these costly metals.
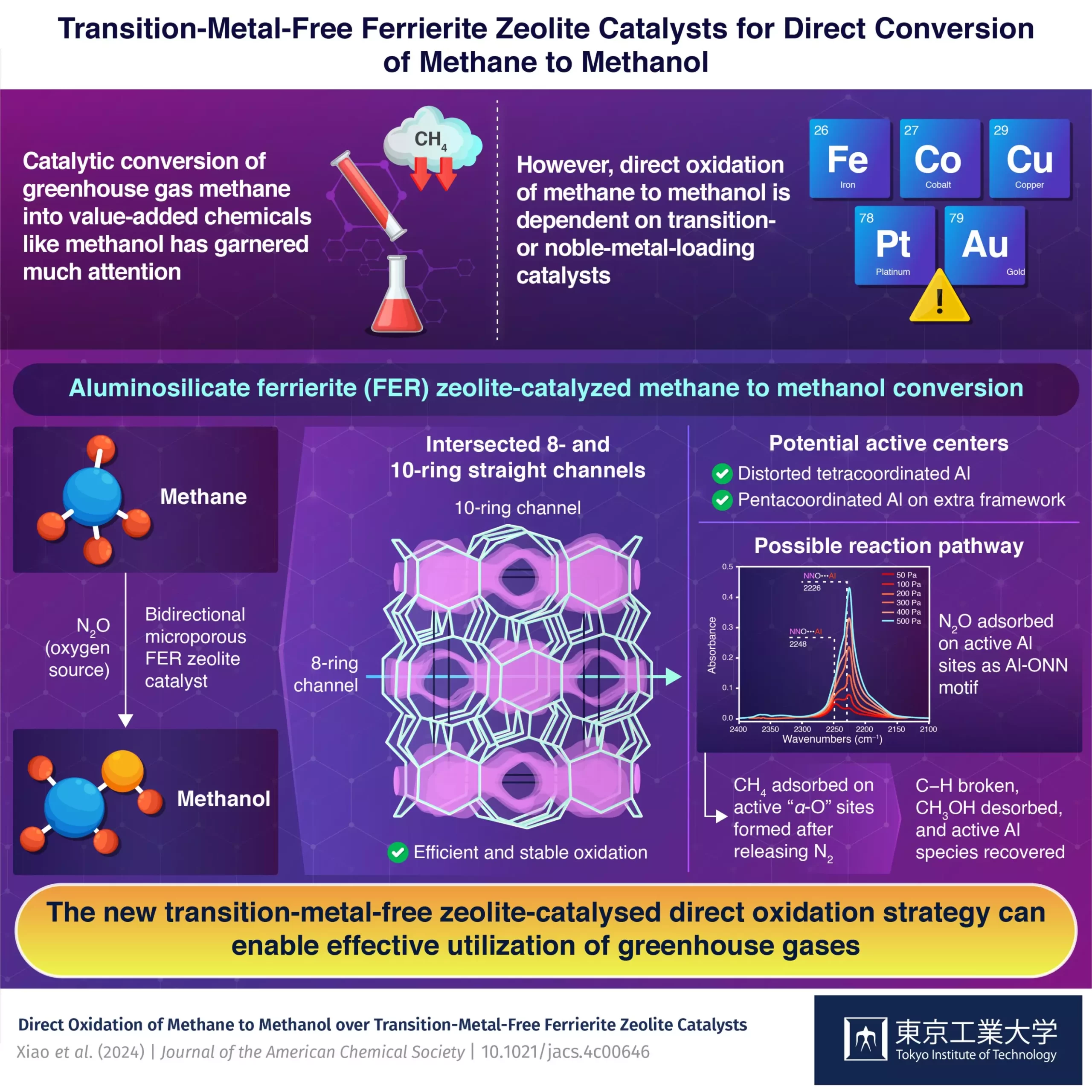
A recent study by a group of researchers at the Nanospace Catalysis Unit of the Institute of Innovative Research at Tokyo Institute of Technology, Japan, led by Associate Professor Toshiyuki Yokoi, introduced a groundbreaking solution using transition-metal-free aluminosilicate ferrierite (FER) zeolite. This 2-dimensional zeolite with unique structural properties enabled the direct oxidation of methane to methanol using nitrous oxide as the source of oxygen.
To understand the active sites within the zeolite catalyst, the researchers conducted Fourier transform infrared spectroscopy (FTIR) and magnetic resonance spectroscopy tests. They discovered potential active centers in the form of distorted tetracoordinated Al in the framework and pentacoordinated Al in the extra framework during the activation, calcination, and reaction processes. The team also analyzed the reaction pathway through nitrous oxide adsorption FTIR spectra and observed different reaction performances under various oxidants.
The transition-metal-free zeolite-catalyzed direct oxidation process demonstrated an impressive methanol production rate of 305 μmol g−1 min−1 with 89% methanol and 10% dimethyl ether selectivity. This performance surpassed many transition metal-loaded catalysts, indicating a significant advancement in the field. The impact of this technology extends beyond the laboratory, paving the way for the utilization of methane and nitrous oxide to produce valuable chemicals on aluminosilicate zeolites with diverse topological structures.
By reducing the reliance on expensive transition metals and offering a more sustainable method for converting methane into methanol, this new technology has the potential to make a positive environmental impact. It contributes to the circular economy by transforming greenhouse gases into valuable materials, thus aligning with the waste-to-wealth movement. Additionally, the synthesis of valuable chemicals from unwanted raw materials can boost the economy and create new opportunities for innovation in the chemical industry.
The development of transition-metal-free aluminosilicate zeolites for the direct oxidation of methane to methanol represents a significant step towards more sustainable and efficient chemical processes. The research conducted by Professor Yokoi and his team highlights the importance of exploring alternative catalyst systems that can reduce costs, improve performance, and contribute to a greener future. This groundbreaking technology opens up new possibilities for generating value from greenhouse gases, shaping the way for a more sustainable and prosperous industrial sector.


Leave a Reply