The study of biological processes and the complex structures of proteins is a crucial field of research. One of the key concepts in this area is chemical diversification of proteins, which involves selectively modifying specific amino acids to create diverse variations of proteins. Cysteine, an amino acid, has been extensively studied and can currently be modified in two ways. However, these methods have limitations such as multistep syntheses and restricted reagent choices. To address these challenges, a groundbreaking technique has been developed by the researchers from the Max Planck Society, led by Tobias Ritter. This article explores their fascinating findings published in Nature Chemistry.
In the current state of research, the synthesis of electrophiles for each desired modification of cysteine requires time-consuming and complex processes. For instance, the creation of a fluorescence probe to track the molecule in biological mixtures necessitates the synthesis of specific electrophiles. Additionally, turning cysteine into a linchpin for chemical diversification involves multistep syntheses, which restrict the introduction of external reagents required for its diversification. Moreover, the preservation of the linchpin’s reactivity during purification processes limits the selection of reagents for functionalization.
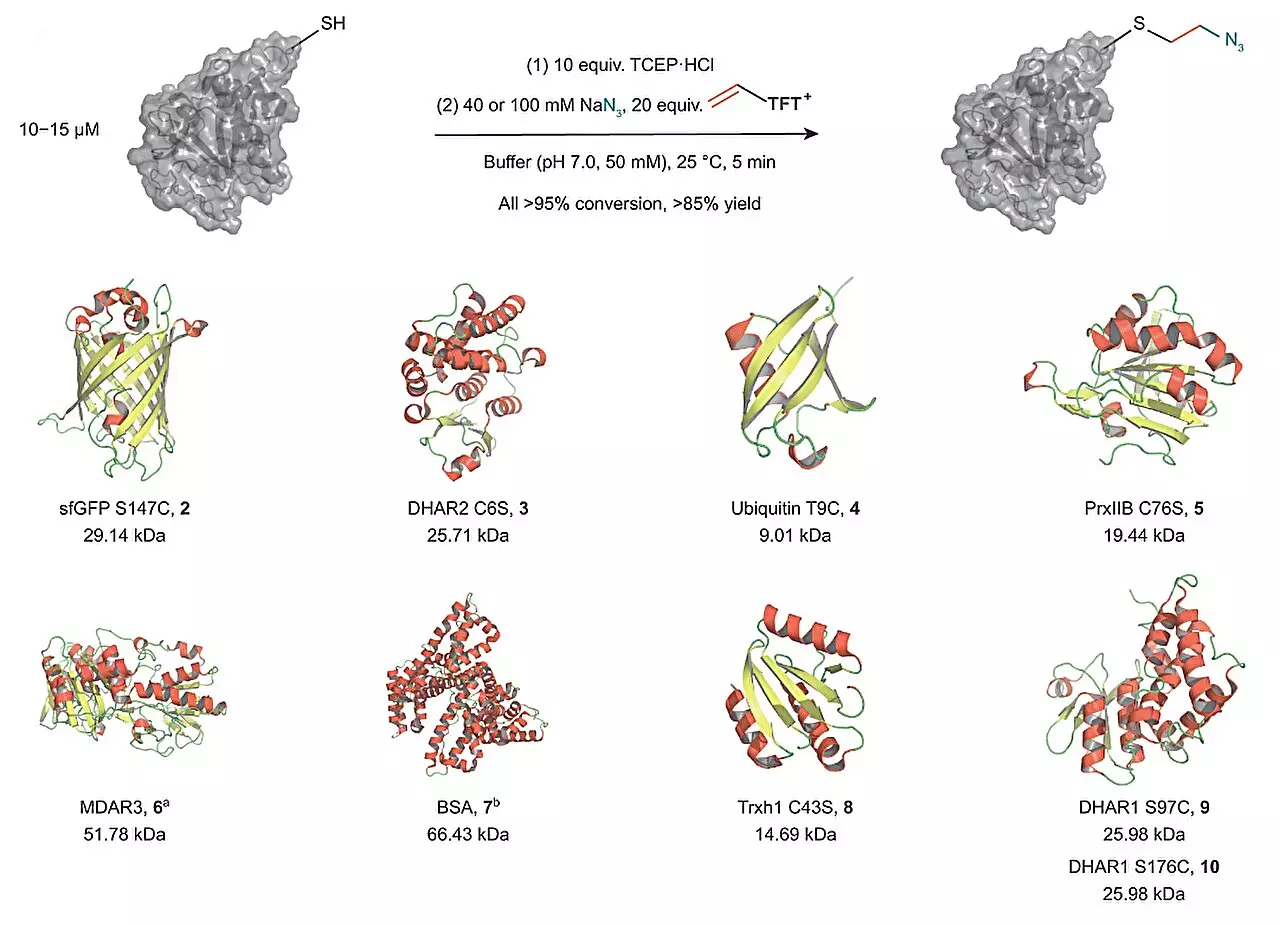
The research group of Tobias Ritter has developed an innovative technique that overcomes the limitations of existing methods. They utilized vinyl thianthrenium salts to transform cysteine into a highly reactive episulfonium electrophile in situ. This one-pot process, based on a single electrophile, offers several advantages. Firstly, it enables the introduction of a highly reactive intermediate without the need for additional steps. Secondly, it allows for a broad diversification of the new intermediate even in the presence of external reagents. This technique revolutionizes the field of chemical diversification of proteins.
The utilization of vinyl thianthrenium salts opens up new possibilities for the modification of cysteine and its interaction with external nucleophiles. This technique enables the scientists to connect cysteine with various other biorelevant functional groups in a single one-pot process. By creating a short and stable ethylene linkage close to the protein’s surface, labels or functionalities can easily be added to alter the chemical environment of the protein. This breakthrough facilitates the study of protein complexes and their altered biological activity.
In addition to the advancements in protein modification, the technique also enhances peptide stability. When no external nucleophiles are added, other amino acids can react with the highly reactive episulfonium intermediate, leading to protein-protein ligation and macrocyclization of linear peptides. This capability increases the stability of peptides against biological degradation, making them more suitable for applications such as drug development.
Another significant contribution of this technique is the synthesis of vinyl thianthrenium salts from ethylene gas. This synthesis approach allows for the production of reagents with different compositions of isotopes. Isotope-labeled compounds possess similar reactivity to non-labeled derivatives but differ slightly in their molecular weight. Mass spectrometry proteomics research can utilize these isotope-labeled compounds to extract quantitative information from whole cellular systems, leading to advanced insights into biological processes.
The technique using vinyl thianthrenium salts developed by Tobias Ritter’s research group proves to be a valuable and broadly applicable tool in the field of chemical biology. Its single electrophile-based process, broad diversification ability, and stability-enhancing properties open new frontiers for protein modification and peptide synthesis. Moreover, the synthesis of reagents with different isotope compositions introduces a new dimension to proteomics research. This breakthrough paves the way for future advancements in understanding biological processes and developing therapeutic interventions.
The Max Planck Society researchers, under the guidance of Tobias Ritter, have made significant strides in the field of chemical diversification of proteins. Their innovative technique utilizing vinyl thianthrenium salts revolutionizes current practices and enables efficient, versatile, and stable protein modification. By overcoming the limitations of multistep syntheses and restricted reagent choices, this groundbreaking approach opens up new avenues for scientific exploration and practical applications in areas such as drug development and proteomics research.


Leave a Reply