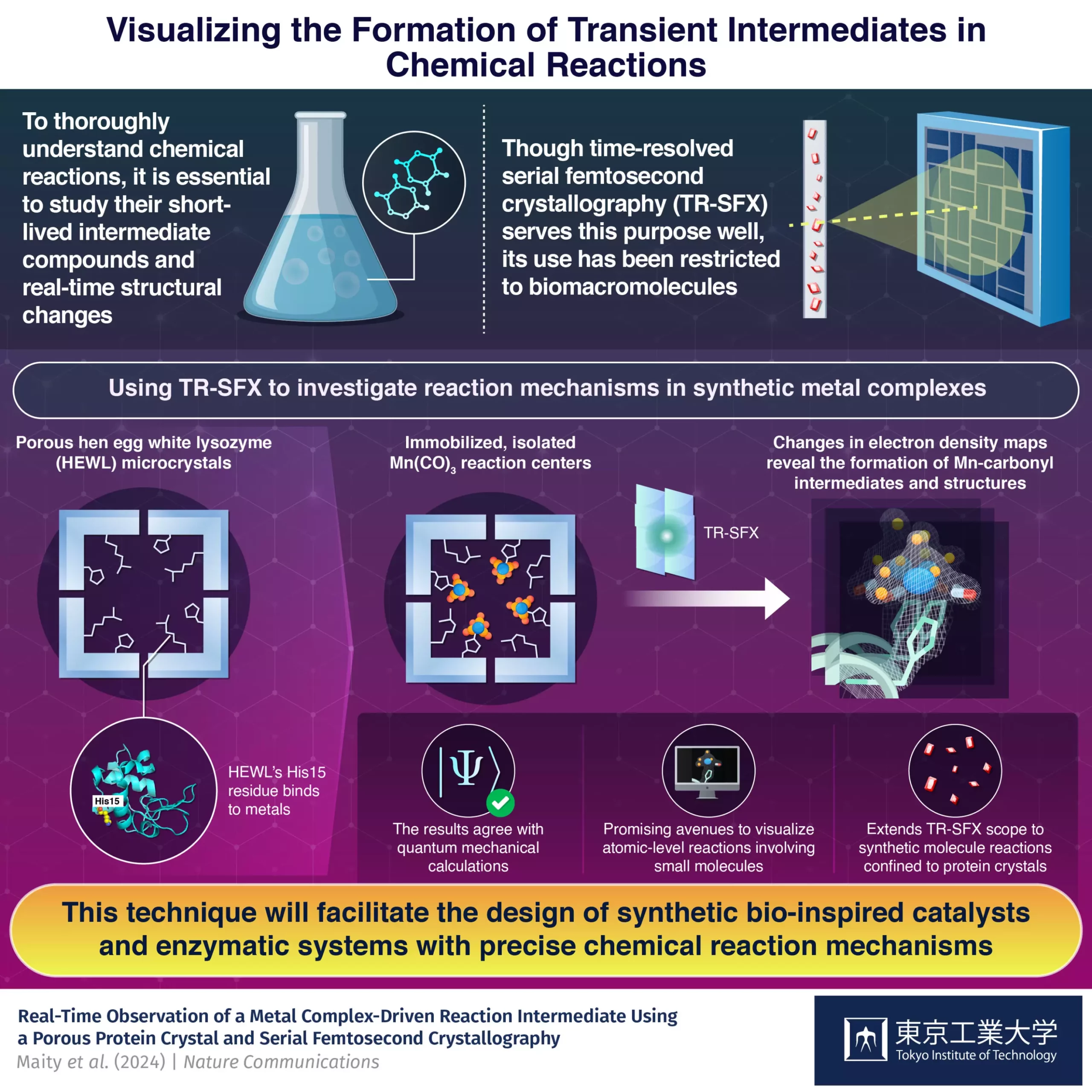
The integration of small synthetic molecules inside protein crystals has emerged as a promising method for studying intermediate compounds formed during chemical reactions. Researchers from Tokyo Tech have successfully visualized reaction dynamics and rapid structural changes by employing this innovative approach with time-resolved serial femtosecond crystallography. Their findings, published in Nature Communications, signify a significant advancement in the understanding of complex chemical reactions.
Most chemical reactions, whether synthetic or biological, involve the formation of short-lived intermediate compounds before yielding final products. Capturing these transient intermediates and analyzing structural changes at the atomic level pose significant challenges to researchers. Traditional methods fall short in visualizing these dynamic processes, necessitating the development of cutting-edge techniques like time-resolved serial femtosecond crystallography (TR-SFX).
While TR-SFX has primarily been utilized for biomacromolecules, a research team led by Professor Takafumi Ueno sought to extend its applications to synthetic compounds. By immobilizing a light-sensitive Mn(CO)3-containing compound inside protein crystals, specifically hen egg-white lysozyme (HEWL), the team was able to analyze the dynamics of carbon monoxide (CO) release. This innovative strategy provided a conducive environment for studying reactions that would typically be challenging to capture.
One of the primary challenges faced by the researchers was the requirement for microcrystals for optimal TR-SFX performance. By leveraging the nanoporous structure of HEWL and its binding affinity to metals, the team devised a solution to immobilize the synthetic compound within the protein crystals. This approach allowed for detailed experimental analysis of the reaction intermediates, enabling a deeper understanding of the chemical processes involved.
Potential Impact on Drug Design and Catalyst Development
The results of this study not only demonstrated the feasibility of studying synthetic metal complexes using protein crystals but also highlighted the potential for designing artificial metalloenzymes with precise mechanisms. This advancement opens up new possibilities for the intelligent design of drugs, catalysts, and enzymatic systems involving non-biological components. By bridging the gap between synthetic and biological chemistry, the method developed by the research team holds promise for driving innovation in reaction design and control.
The integration of small synthetic molecules inside protein crystals represents a significant step forward in the field of chemical reaction studies. The successful visualization of reaction dynamics and intermediate compounds using TR-SFX showcases the potential for advancing various scientific disciplines, including energy generation, catalysis, and medicine. By pushing the boundaries of traditional crystallography methods, researchers are paving the way for novel approaches to designing functional materials and understanding complex chemical processes at the molecular level.


Leave a Reply