In the field of photochemistry, the search for highly reducing or oxidizing photocatalysts has always been a significant challenge. Previous studies have mainly focused on transition metal complexes with precious metals, such as ruthenium and iridium. However, these complexes come with their limitations, including the need for high energy light for excitation and the high cost associated with precious metals. In a groundbreaking development, a team of researchers at Johannes Gutenberg University Mainz has discovered a new molecular system based on the element manganese that addresses these limitations. The study, published in the journal Nature Chemistry, introduces a highly efficient and cost-effective photocatalyst with huge potential for various applications.
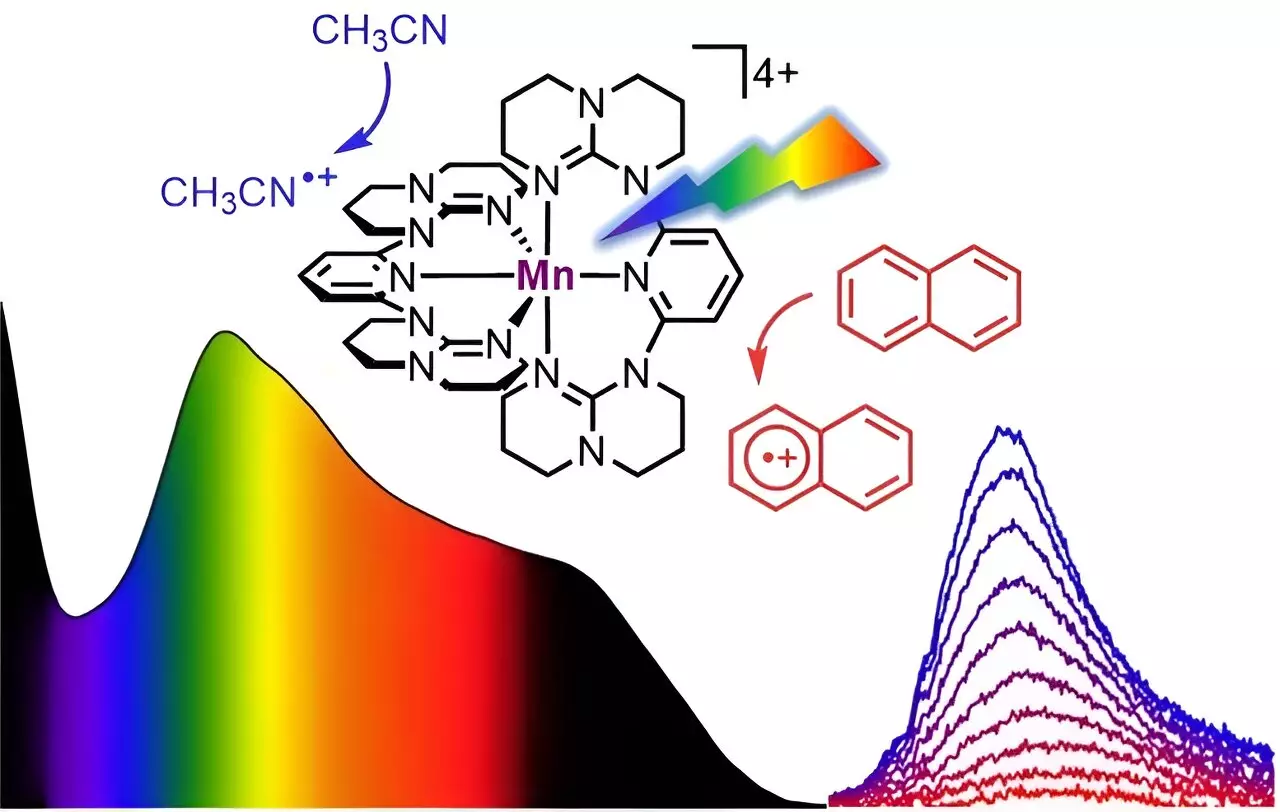
One of the key advantages of the newly developed molecular system is its reliance on manganese, which is the third most abundant metal on Earth. Unlike precious metals, manganese is widely available and relatively cheap, making it a groundbreaking alternative for highly efficient photocatalysts. The team at Johannes Gutenberg University Mainz successfully designed a soluble manganese complex that exhibits panchromatic absorption, absorbing all visible light from blue to red and even parts of the near-infrared light. This broad absorption range is reminiscent of the dark color of Braunstein or manganese dioxide, a natural mineral.
The most remarkable observation in this study is the NIR-II luminescence emitted by the “molecular Braunstein” after excitation with visible or near-infrared light. This molecular manganese system surpasses the behavior of even noble metals in terms of emission in this energy region. Additionally, the “molecular Braunstein” demonstrates its extraordinary oxidizing power by oxidizing various organic substrates, including challenging aromatic molecules with high oxidation potentials like naphthalene, toluene, and benzene. Even solvents that are typically stable can be attacked by this superphotooxidant when excited by LED light.
To understand the underlying mechanisms of this novel molecular system, the researchers employed ultrafast spectroscopic techniques using laser pulses with sub-picosecond time resolution. These techniques revealed two distinct photoactive states: a short-lived but highly oxidizing high-energy state and a longer-lived moderately oxidizing lower-energy state. The former interacts with solvent molecules in close proximity before light excitation, while the latter state exists long enough to attack aromatic substrates after diffusional collision. This unique behavior is known as static and dynamic quenching of the excited states and provides valuable insights into the photoinduced processes.
To gain a more comprehensive understanding of the involved excited states, the researchers resorted to quantum chemical calculations, which were only possible due to the computing power of supercomputers. The calculations, performed using the MOGON and ELWETRITSCH supercomputers in Rhineland-Palatinate, helped to refine the models of the excited states and align them with the spectroscopic results. This quantum chemical study was instrumental in unraveling the detailed picture of the photoinduced processes involved in this groundbreaking molecular system.
With the development of this novel molecular manganese system, scientists are poised to revolutionize light-driven reactions in various fields. This breakthrough not only offers a sustainable and affordable alternative to the expensive ruthenium and iridium compounds commonly used in photocatalysis but also opens up possibilities for new reaction and substrate classes that were previously inaccessible. Equipped with their own state-of-the-art ultrafast laser system, advanced supercomputing capabilities, and a team of talented Ph.D. students, the researchers at Johannes Gutenberg University Mainz are committed to furthering the progress in developing a more sustainable and efficient photochemistry.
The discovery of a new molecular system based on the abundant and affordable metal manganese is a game-changer in the field of photocatalysis. By harnessing the unique properties of manganese, such as panchromatic absorption and NIR-II luminescence, the development of highly efficient and cost-effective photocatalysts is within reach. This breakthrough offers immense potential for various applications and brings us one step closer to a more sustainable future in photochemistry.


Leave a Reply