Scientists from the Department of Energy’s SLAC National Accelerator Laboratory have recently made significant progress in understanding and producing nitroxide, a molecule with promising potential in the biomedical field. While the physiological effects of nitric oxide (NO) have been widely studied, its lesser-known cousin, nitroxide (HNO), has remained largely unexplored. This research, published in the Journal of the American Chemical Society, was a collaborative effort between the teams at SLAC’s Linac Coherent Light Source (LCLS) X-ray laser and Stanford Synchrotron Radiation Lightsource (SSRL).
Nitroxide exhibits many of the same physiological effects as nitric oxide, including its ability to fight germs, prevent blood clots, and relax and dilate blood vessels. However, it also possesses additional therapeutic properties, such as efficacy in treating heart failure, more potent antioxidant activity, and wound healing. Despite these potential applications, nitroxide is not a chemically stable compound, making targeted delivery methods crucial for future biomedical use.
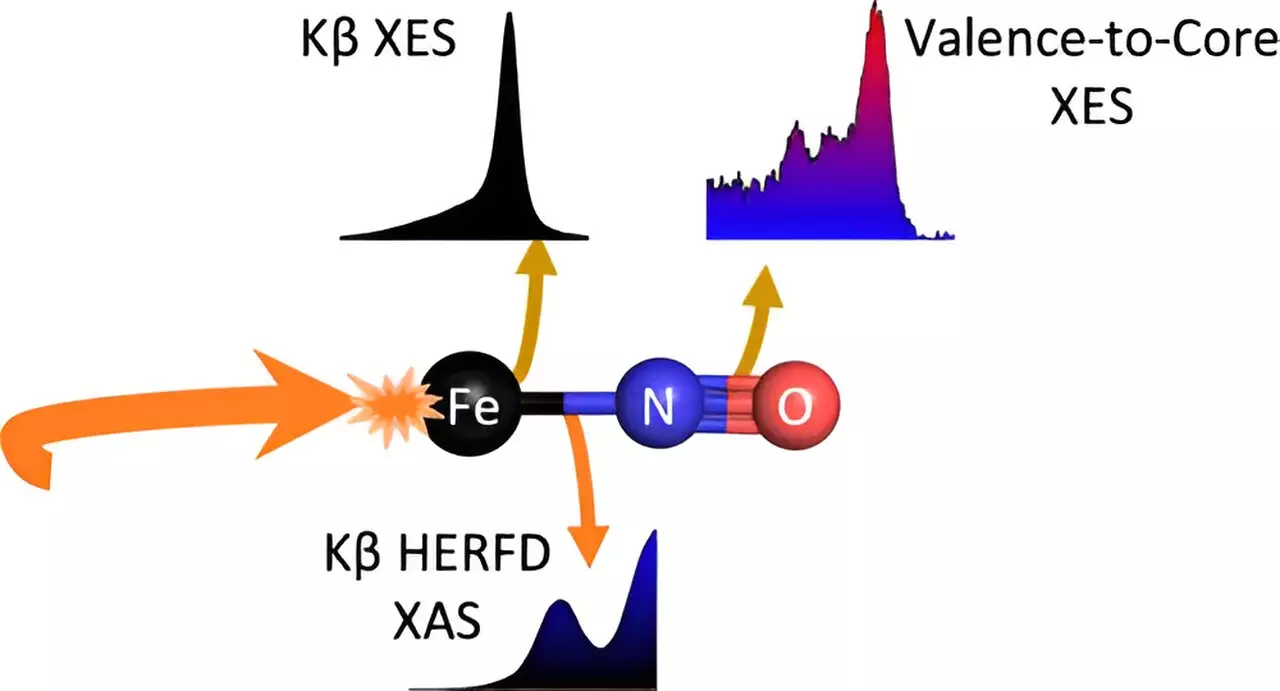
The research team focused on a specific molecule, an iron-nitrosyl complex (Fe-NO), in their quest to understand the intricate properties of the Fe-NO bond and unlock the complexities of nitroxide production. By exposing this molecule to optical light, they discovered that the bond could be broken, potentially resulting in the production of nitroxide. While this research has fundamental implications, one of its main objectives is to provide a foundation for future therapeutic technologies based on optimizing similar molecules for medicine. The vision is to develop a molecule that can release HNO when needed in the body, and subsequently, apply light to release it for its therapeutic properties.
One of the challenges faced by the research team was the ambiguous distribution of electrons between the iron atom and the nitrosyl ligand within the Fe-NO complex. This ambiguity limits the amount of information that can be obtained using traditional methods. To overcome this limitation, the scientists employed advanced X-ray spectroscopic techniques at SSRL, enabling them to gain deeper insights into the chemical properties of the molecule and its bond. This approach provided a more comprehensive understanding of the Fe-NO system and its response to light.
Moving forward, the researchers plan to further investigate the bond-breaking process and optimize the production of nitroxide or nitric oxide. They also intend to explore the possibility of replacing iron with other metals to gain a better understanding of the photoproduction process. While this study shed light on the starting molecule and its final products after light exposure, there are still many nuances to explore regarding the release of nitroxide instead of nitric oxide. Furthermore, the researchers aim to structurally tune the system to selectively produce either molecule, opening up new possibilities for biomedical applications.
The information gained from this study highlights the power of the approach used and serves as a blueprint for future investigations. At the LCLS, scientists will be able to capture real-time snapshots of the nitroxide photogeneration process, further advancing our understanding of these types of molecules. This research holds great promise for the medical community and patients who may benefit from its future applications. While the use of light on these molecules to treat serious cardiovascular conditions is still a distant goal, the fundamental insights gained from this research lay a substantial foundation for applied research in the future.
The study conducted by scientists from SLAC National Accelerator Laboratory provides valuable insights into the production and potential applications of nitroxide in the biomedical field. By exploring the properties of an iron-nitrosyl complex and its response to light, the researchers have made significant strides in understanding the intricate bond-breaking process. This research not only sets the stage for future investigations but also paves the way for the development of therapeutic technologies based on nitroxide. The biomedical community eagerly anticipates the translation of these fundamental findings into practical applications that can improve patient outcomes and revolutionize the field of medicine.


Leave a Reply