In a world where environmental sustainability is becoming increasingly crucial, the field of chemistry is also undergoing a transformation towards greener practices. Chemists from Yokohama National University have recently made a groundbreaking discovery that could revolutionize the way chemical reactions are conducted, paving the way for a more eco-friendly future.
The Need for Green Chemistry
Traditional chemical synthesis methods often involve the use of hazardous chemicals and wasteful processes that contribute to environmental pollution and safety concerns. As a result, there is a growing demand for sustainable chemical synthesis strategies that are more efficient and environmentally friendly. The development of catalysts that can enable greener chemical reactions is a significant step towards achieving this goal.
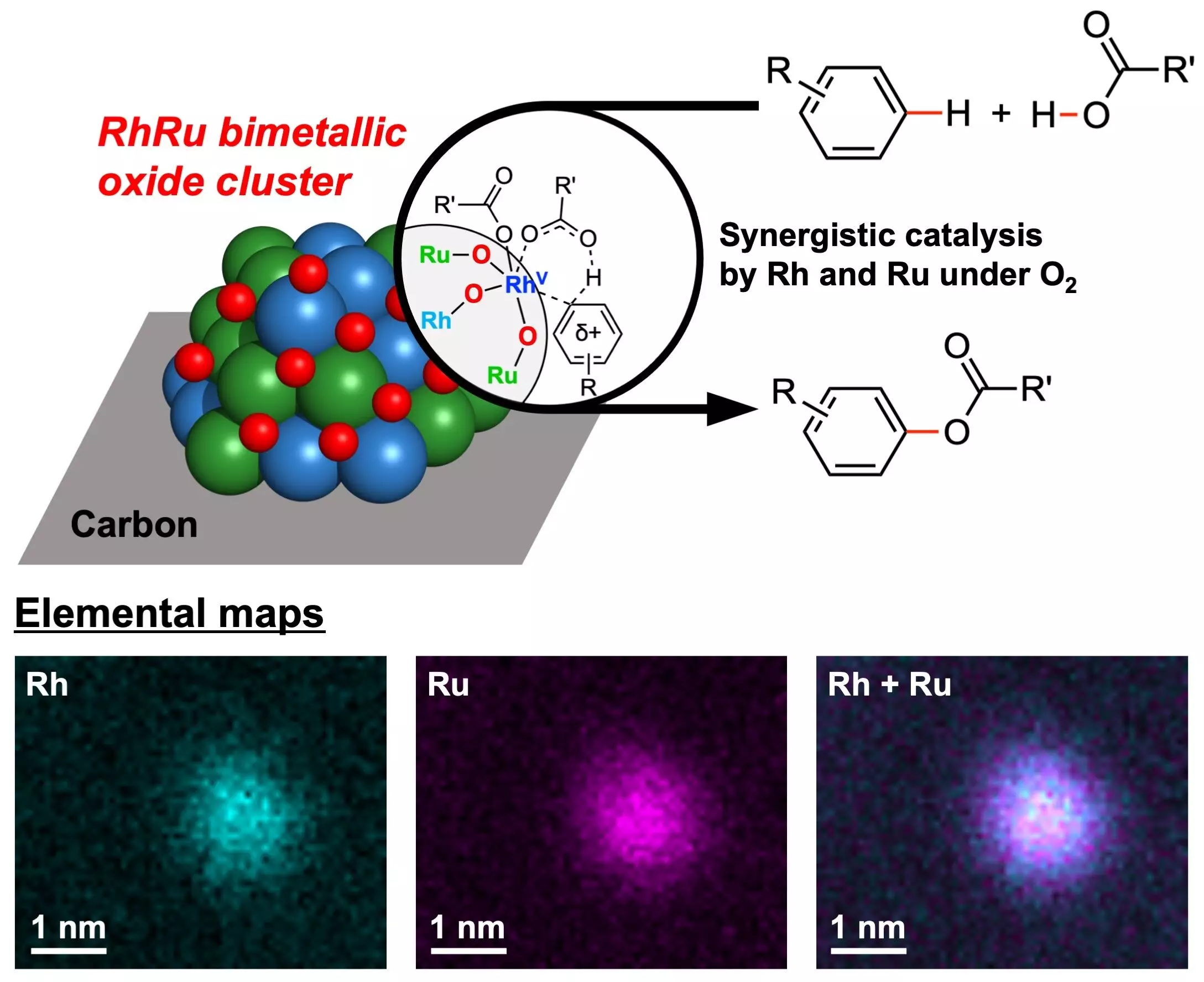
The research team at Yokohama National University has successfully created novel catalysts containing two noble metals, Rhodium (Rh) and Ruthenium (Ru), that demonstrate exceptional efficiency in ester-producing chemical reactions. These catalysts, known as RhRu bimetallic oxide clusters (RhRuOx/C), utilize oxygen as the sole oxidant, eliminating the need for hazardous oxidizing agents traditionally used in Cross-Dehydrogenative Coupling (CDC) reactions.
The Role of Noble Metals
Noble metals have long been valued for their catalytic properties, and the combination of Rhodium and Ruthenium in the newly developed catalysts has proven to be highly effective in promoting C–H bond activation reactions. These reactions play a crucial role in organic synthesis and industrial chemistry, making the catalysts versatile for various applications in chemical production.
One of the key advantages of the RhRu bimetallic oxide clusters is their ability to utilize molecular oxygen as the sole oxidant. Unlike traditional oxidants that are toxic and environmentally harmful, oxygen is non-toxic, abundant, and environmentally benign. This not only reduces the environmental impact of the chemical reactions but also minimizes the production of harmful byproducts, with water being the only byproduct generated.
The development of these innovative catalysts opens up new possibilities for sustainable and efficient chemical synthesis practices. By harnessing the power of noble metals and oxygen, the researchers have demonstrated a promising path towards greener chemistry. The next steps involve exploring the use of these catalysts in other important chemical reactions, with the ultimate goal of establishing more environmentally friendly chemistry practices.
The breakthrough in catalyst development by the chemists at Yokohama National University represents a significant advancement in the field of green chemistry. By creating catalysts that can facilitate ester-producing chemical reactions using oxygen as the sole oxidant, the researchers have demonstrated a more sustainable and efficient approach to chemical synthesis. This achievement not only highlights the potential for greener chemistry practices but also sets the stage for further innovation in the field of catalysis and environmental sustainability.


Leave a Reply