The quest for sustainable water disinfection treatments has led researchers from the University of Pittsburgh, Drexel University, and Brookhaven National Laboratory to delve into the world of scalable electrochemical ozone production (EOP) technologies. With the potential to revolutionize water disinfection processes in both urban centers and rural communities, EOP presents a promising alternative to traditional chlorine treatments. However, the intricate mechanisms behind EOP at a molecular level remain largely unknown, posing challenges in developing efficient, cost-effective, and sustainable solutions.
In a recent publication in ACS Catalysis, the collaborative research team shed light on the interplay between catalyst corrosion and homogeneous reactive oxygen species in electrochemical ozone production. Led by Drexel Ph.D. student Rayan Alaufey, the study involved contributions from esteemed researchers such as Maureen Tang, Andrew Lindsay, Tana Siboonruang, Ezra Wood, John A. Keith, Lingyan Zhao, and Qin Wu. The investigation aimed to uncover the underlying factors that influence the efficacy and stability of EOP catalysts, particularly nickel- and antimony-doped tin oxide (Ni/Sb–SnO2, or NATO), known for its promising disinfection capabilities.
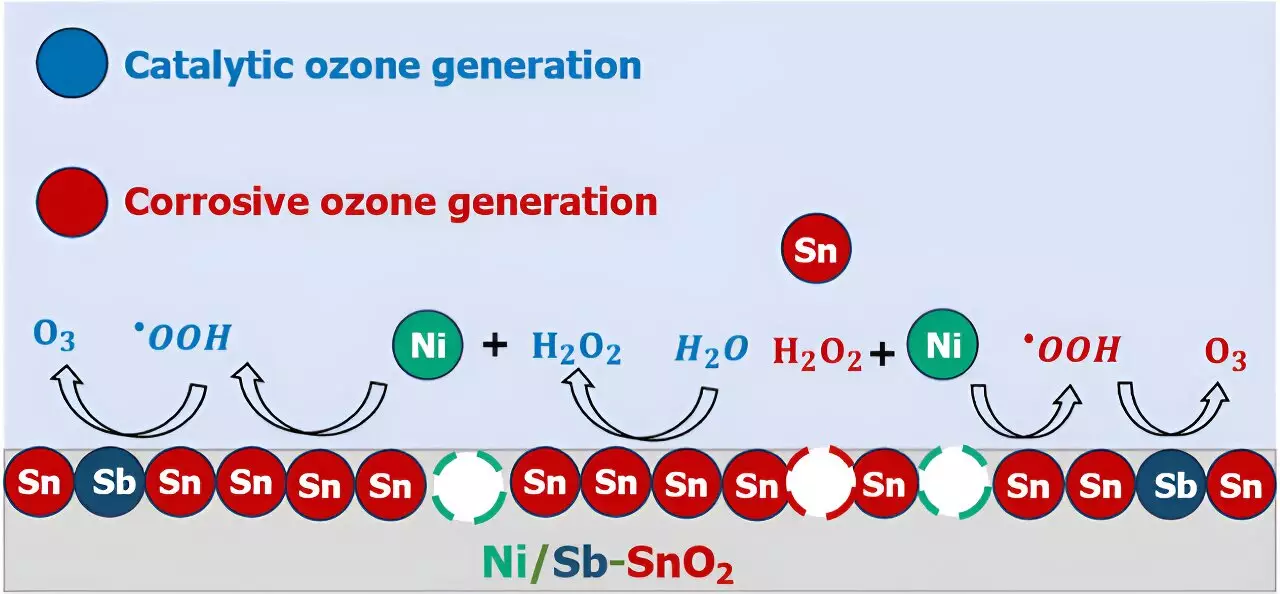
A pivotal question addressed by the researchers was the critical roles that individual atoms within NATO play in facilitating EOP. Through a combination of experimental electrochemical analyses, mass spectrometry, and computational quantum chemistry modeling, the team constructed an intricate atomic-scale narrative that elucidated the ozone generation process on NATO electrocatalysts. Surprisingly, the findings unveiled a dual mechanism where nickel leaching from the electrodes via corrosion contributed to ozone formation, highlighting the complex interplay between catalyst degradation and ozone production.
The revelations regarding corrosion-induced nickel leaching and its impact on ozone generation underscored the necessity of comprehending catalyst behavior on a fundamental level. As Keith emphasized, distinguishing between authentic catalytic processes and secondary reactions occurring in the vicinity of the catalyst is crucial for advancing EOP technologies. By acknowledging the presence of corrosion and solution-phase reactions, researchers can chart a path towards refining EOP catalysts and optimizing electrochemical oxidation processes.
In their concluding remarks, the researchers emphasized the significance of resolving fundamental technological obstacles to propel the widespread adoption of EOP and other electrochemical oxidation techniques. While acknowledging the efficacy of electrochemical water treatment on a smaller scale, the pressing need for innovative catalysts with enhanced resistance to corrosion and improved efficiency remains paramount. Keith’s vision of exploring novel atomic combinations in materials holds the key to unlocking the full potential of sustainable and economically viable EOP solutions.
In essence, the journey towards sustainable water disinfection through electrochemical ozone production is rife with challenges and opportunities. By unraveling the intricate mechanisms governing catalyst behavior and ozone generation, researchers are paving the way for transformative advancements in water treatment technologies. As the quest for innovative EOP catalysts continues, the promise of scalable, cost-effective, and environmentally friendly solutions beckons, offering a glimmer of hope for a future where clean water is a global reality.


Leave a Reply