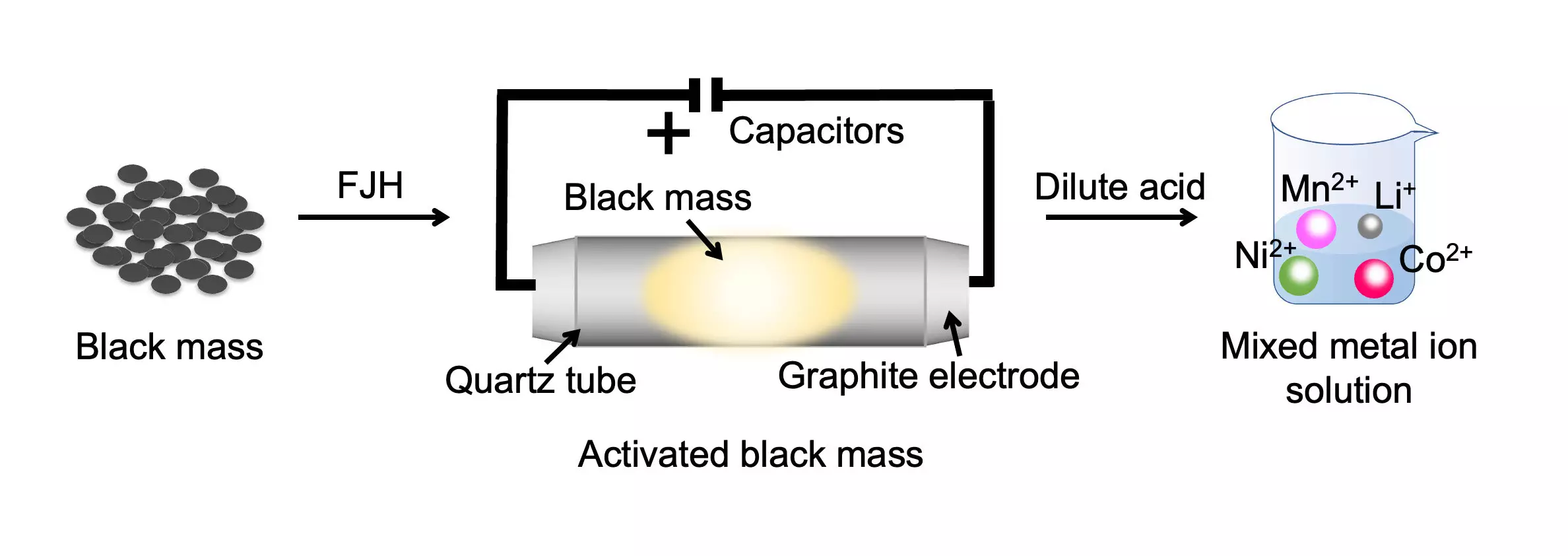
The demand for valuable metals used in batteries is set to soar in the coming decades, as clean energy technologies continue to expand. To meet this demand sustainably, scientists at Rice University have developed an innovative battery recycling process. This groundbreaking method removes the inert layer on battery metals and lowers their oxidation state, making them soluble in low-concentration acid. By employing the signature Joule-heating technique, which rapidly heats the combined cathode and anode waste to temperatures above 2,100 degrees Kelvin, the researchers achieved a remarkable metal recovery yield exceeding 98% from various types of mixed battery waste.
Traditionally, the battery recycling industry has struggled to deal with the combined cathode and anode waste, known as “black mass.” However, this new approach developed by the Rice University team significantly reduces the environmental impact of spent battery processing. Not only does it reduce secondary waste streams from the contaminated leaching solutions, but it also slashes the recycling process duration by almost 100-fold. Instead of taking 24 hours, the same amount of materials can now be dissolved in less than 20 minutes. This breakthrough has the potential to revolutionize battery waste recycling by cutting energy, water, and acid consumption, as well as lowering carbon dioxide emissions.
One of the key advancements of this method is focused on improving the leaching kinetics of the metals. By decomposing the passivated layer and regulating the metal valence state for the first time, the team achieved enhanced leaching kinetics. This development not only increases the efficiency of metal recovery but also lays the foundation for future advances in the industry. This breakthrough technology could help fuel the rapid growth of the battery recycling business, which is projected to expand as more electric vehicles and electronic devices reach the end of their lifecycle.
Battery recycling not only helps mitigate the environmental impact of mining but also makes economic sense. Many lithium-ion batteries contain higher concentrations of metals like cobalt and nickel than natural ores. By recycling spent batteries, these valuable metals can be recovered and reused, reducing the need for new mining operations. The current recycling capacity is insufficient, with only 5% of batteries being recycled. However, as electronic waste continues to increase at an annual rate of 9%, implementing more efficient recycling processes becomes crucial.
The Rice University research team’s flash method offers a more economical and environmentally friendly solution. It involves subjecting the black mass to a rapid heating process, which separates the critical metals. The separated metals can then be easily dissolved using low-concentration hydrochloric acid. The flash treatment liberates the metals, making them more soluble and reducing the need for large quantities of acid. This innovation not only improves the economics of battery recycling but also simplifies the overall process.
The new battery recycling process developed by Rice University has the potential to transform the industry. It not only addresses the pressing need for sustainable metal sourcing but also contributes to the mass production of electric vehicles at a more competitive cost by lowering battery production expenses. This breakthrough technology represents a significant step towards a greener and more sustainable future, where valuable metals can be recycled and reused, reducing our reliance on mining and minimizing environmental harm. As clean energy technologies continue to advance, battery recycling will play an increasingly crucial role in achieving a more sustainable and circular economy.


Leave a Reply