Solar energy has been gaining momentum as the fastest growing renewable energy source in the United States, fueled by advancements in technology that enhance the conversion of solar light into electricity. However, researchers are also looking at harnessing light for chemical reactions. The chemical industry plays a critical role in producing everyday consumer and industrial products, but it heavily relies on non-renewable sources for energy. According to the 2022 U.S. Department of Energy Industrial Decarbonization Roadmap, the chemicals and petrochemicals sectors contribute to 40% of industrial energy use and emissions in the U.S. The University of Illinois Urbana-Champaign is at the forefront of exploring light-induced chemistry, with Professor Christy Landes leading a team of researchers dedicated to this innovative pursuit.
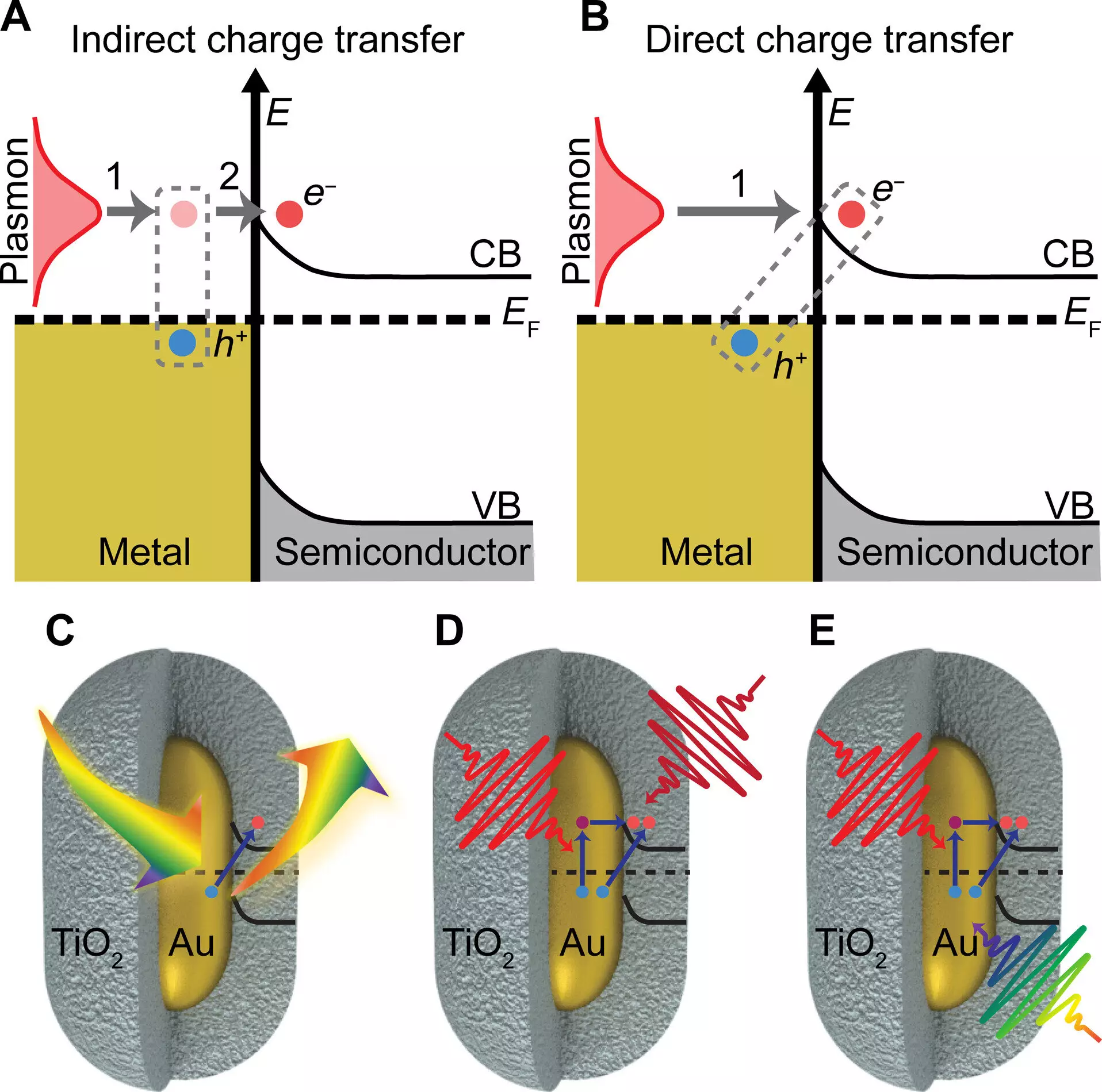
In a recent study published in Science Advances, the research team from Illinois unveiled a novel mechanism of charge transfer that surpasses traditional methods in both speed and efficiency. By leveraging plasmonic gold nanoparticles, which are 1/1000th the width of a human hair, the researchers were able to enhance charge transfer to a semiconductor made of titanium oxide shells. Through their experiments with different light colors, the team determined the optimal conditions for maximizing charge transfer. This breakthrough holds immense promise in improving light-harvesting technologies for generating currents and driving chemical reactions.
Professor Stephan Link, a co-lead author of the study, emphasized the significance of understanding the role of plasmon in facilitating charge transfer. The study confirmed that exciting the plasmon with light resulted in a considerable boost in charge transfer efficiency. The researchers reported an impressive electron transfer efficiency of 44 ± 3% from gold nanorods to titanium oxide shells when excited on resonance. Half of this efficiency was attributed to direct interfacial charge transfer mediated by plasmon excitation. This finding underscores the crucial role of plasmonic nanoparticles in enhancing charge separation and transfer.
Gold nanoparticles exhibit unique properties at the nanoscale, including tunable color and the ability to catalyze reactions. The research team discovered that the charge transfer efficiency of gold particles significantly increased when they absorbed light matching their color. This observation led to the realization that the plasmon of gold nanoparticles acts as an additional channel for boosting charge transfer. By exploiting this plasmon-induced pathway, the researchers were able to circumvent heat generation, which can impede the desired charge transfer process. This strategic manipulation of plasmon excitation opens up new possibilities for achieving efficient light-driven chemistry.
The study also shed light on a long-neglected process known as chemical interface damping of plasmons, originally proposed in the 1990s. Professor Landes expressed excitement over the validation of this old theory in the context of contemporary research on plasmonics and photocatalysis. By integrating multiple imaging and spectroscopy techniques, the research team successfully elucidated the mechanisms underlying plasmon-induced charge transfer. This multidisciplinary approach allowed for a comprehensive understanding of chemical interface damping and its implications for advancing light-driven chemical reactions.
The groundbreaking research conducted at the University of Illinois Urbana-Champaign highlights the transformative potential of solar energy in driving efficient chemical reactions. By harnessing the unique properties of plasmonic nanoparticles and leveraging light-induced charge transfer mechanisms, researchers are paving the way for sustainable and energy-efficient chemical processes. The integration of solar energy into chemistry represents a promising avenue for reducing reliance on non-renewable resources while expanding the horizons of scientific innovation.


Leave a Reply