In the pursuit of combating climate change, scientists at the Department of Energy’s Oak Ridge National Laboratory have taken a significant step forward in understanding the process of direct air capture (DAC) of carbon dioxide from the atmosphere. DAC aims to achieve negative emissions, where the amount of carbon dioxide removed from the Earth’s envelope of gases exceeds the amount emitted. This research, recently published in Cell Reports Physical Science, delves into the foundational steps of carbon dioxide sequestration and highlights the critical role of kinetics, thermodynamics, and molecular interactions.
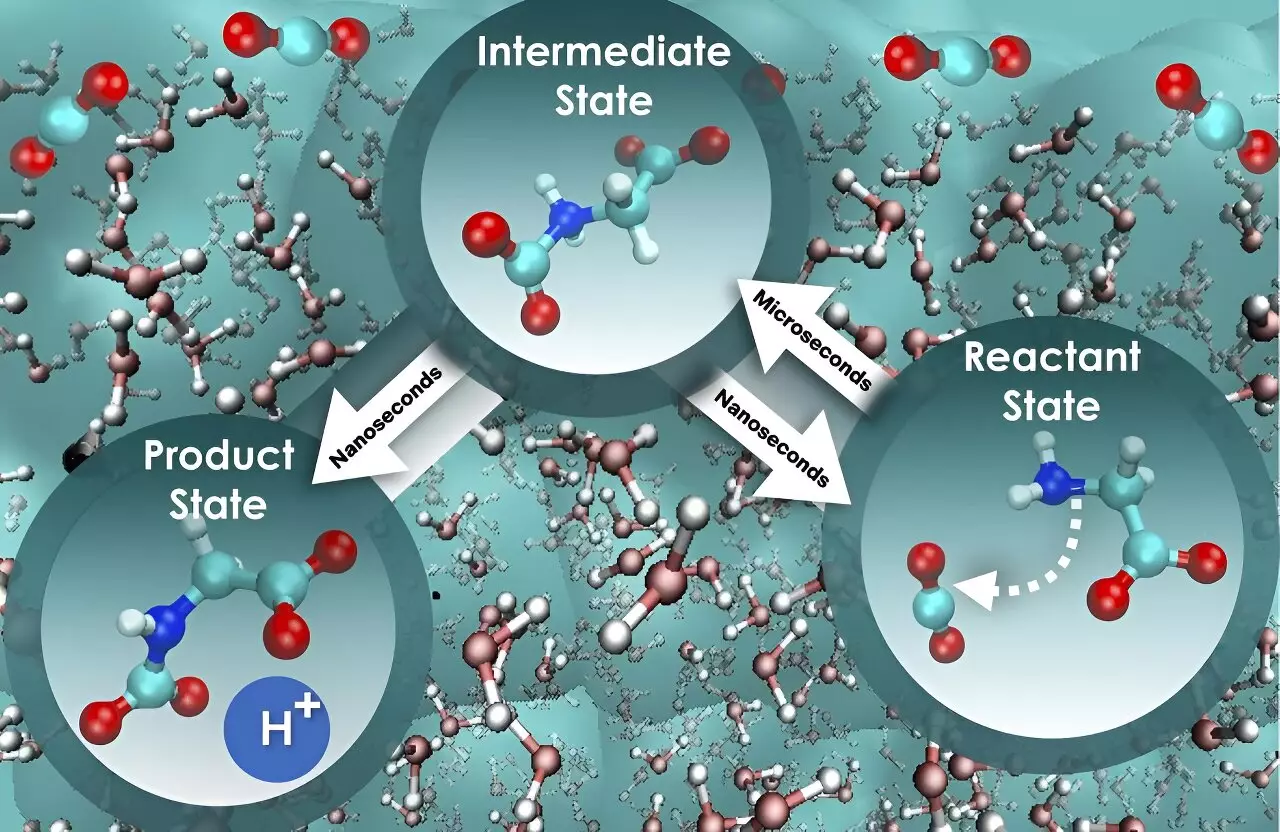
Chemical reactions in water are inherently complex, particularly when the motion of water molecules plays a substantial role. According to Santanu Roy, one of the researchers involved in the study, water molecules and chemicals engage in a dynamic interaction similar to a coupled dance, which can either marginally or significantly slow down a reaction. Understanding these dynamic interactions, termed nonequilibrium solvent effects, is crucial for gaining a comprehensive understanding of reaction kinetics and the speed at which they occur.
The researchers discovered that focusing solely on the free energy barrier, which represents the energy threshold that must be overcome for a system to transition from one state to another, provides an oversimplified view that hinders a full understanding of the rate at which carbon dioxide is absorbed. By employing a more comprehensive approach, the study revealed intriguing insights. The initial step of glycine interacting with carbon dioxide was found to be nearly 800 times slower compared to the subsequent step of a proton release, which leads to the formation of a mixture of product states responsible for holding the absorbed carbon dioxide. The constancy of the free energy barrier for both steps sets them apart in terms of speed, offering a potential pathway to enhance the efficiency of carbon dioxide absorption and separation.
The extensive molecular dynamics simulations employed in this study were limited by their short time and length scales, as well as their high computational costs. To address these limitations, the researchers propose combining a machine-learning approach with highly accurate simulations. By developing interatomic interaction potentials based on deep neural networks, molecular simulations can be performed at large scales with significantly reduced computational costs. This advancement would enable researchers to explore the effects of macroscopic factors such as temperature, pressure, and viscosity on DAC, enhancing our understanding of the molecular-level kinetics of carbon dioxide capture.
Overall, this study’s findings shed light on the intricate workings of DAC and underscore the importance of kinetics, thermodynamics, and molecular interactions in the removal of carbon dioxide from the atmosphere using aqueous amino acids. As our understanding of these mechanisms improves, the feasibility of deploying large-scale DAC technology becomes more promising. Around the world, several DAC projects are currently in various stages of research, testing, and development. Combined efforts in advancing DAC technology hold the potential to contribute significantly to global efforts in mitigating climate change.
The research conducted at the Department of Energy’s Oak Ridge National Laboratory provides valuable insights into the complexities involved in direct air capture of carbon dioxide. By investigating the foundational steps of carbon dioxide sequestration and understanding the dynamic interactions in liquid solutions, scientists are taking steps towards achieving negative emissions. This newfound knowledge will pave the way for more effective carbon dioxide absorption methods and ultimately contribute to a cleaner and more sustainable future.


Leave a Reply