In a breakthrough collaboration, two brothers who are scientists in unrelated fields have joined forces to address a chemical problem concerning the use of silicon in electronic devices. Dr. Tamim Darwish, leader of the National Deuteration Facility (NDF), proposed the idea of deuterating silicon to his brother, Dr. Nadim Darwish, a Senior Lecturer specializing in molecular electronics at Curtin University. This unique partnership aimed to explore the potential improvements in silicon properties through the replacement of hydrogen with deuterium.
Dr. Tamim Darwish, with his extensive knowledge of deuterium, an isotope of hydrogen, is well-versed in its unique properties. Deuterium is commonly used to replace hydrogen in molecules, and the NDF at ANSTO (Australian Nuclear Science and Technology Organisation) is a leading facility focused on deuteration for research purposes. The NDF offers tailored deuterated molecules and labeling techniques as per researchers’ requirements.
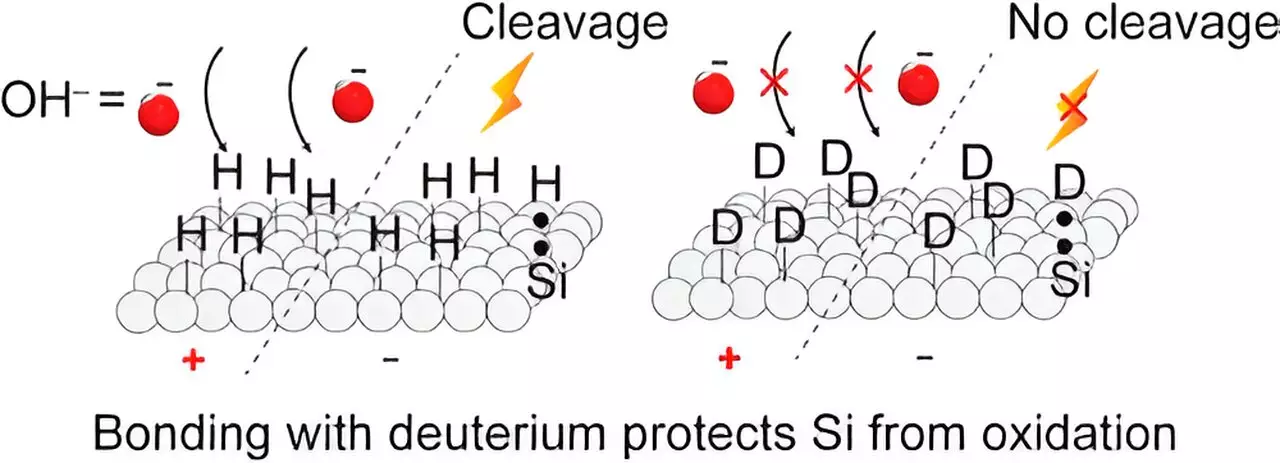
The research conducted by the Darwish brothers and their colleagues, published in ACS Applied Materials & Interfaces, highlights the significant improvements in silicon properties when hydrogen atoms are replaced with deuterium on the surface layer. The integration of silicon and organic molecules in various applications, such as sensors, solar cells, power generation, and molecular electronic devices, has garnered immense interest in recent years. However, a major challenge faced in this technology is the susceptibility of silicon and hydrogen (Si−H surfaces) to oxidation. This oxidation adversely affects the mechanical and electronic stability of these devices.
The research findings demonstrate that by substituting hydrogen with deuterium, creating Si−D surfaces, the silicon surfaces become significantly more resistant to oxidation under both positive and negative voltages. Si−D surfaces exhibit enhanced stability against oxidation, and their electrical characteristics remain consistent compared to Si−H surfaces. Consequently, the investigators recommend the use of Si−D surfaces in applications that require non-oxidized silicon surfaces, such as electrochemical biosensors, silicon-based molecular electronic devices, and silicon-based triboelectric generators.
Broader Implications and Future Applications
The notable surface isotope effects identified in this study have far-reaching implications for the design of silicon-based devices, molecular electronics, and power generation devices reliant on silicon. Furthermore, these findings influence the interpretation of charge transport characteristics in similar devices. Incorporating deuterium into silicon surfaces opens up a new realm of possibilities for enhancing the stability and performance of electronic devices, unlocking their full potential in various industries.
The collaboration between the two Darwish brothers, with their expertise in deuteration and molecular electronics, has yielded promising results. By deuterating silicon, the researchers have discovered a solution to the daunting challenge of oxidation faced by silicon-based devices. The replacement of hydrogen atoms on the silicon surface with deuterium has led to enhanced stability, improved electrical characteristics, and increased resistance to oxidation. This breakthrough has far-reaching implications for the development and optimization of silicon-based devices, paving the way for advancements in electronics, power generation, and other innovative applications.


Leave a Reply