Solid materials have traditionally been viewed as rigid and immobile. However, scientists are challenging this notion by exploring ways to incorporate moving parts into solids. This groundbreaking approach opens up opportunities for the development of exotic new materials, including amphidynamic crystals, which contain both rigid and mobile components. By controlling molecular rotation within the material, scientists can alter its properties, leading to exciting possibilities in various fields of science and engineering.
One of the major challenges in achieving motion in crystals, and solids in general, is the tightly packed nature of their structure. This compact arrangement restricts dynamic motion to molecules of a limited size. Overcoming this obstacle requires ingenious solutions and innovative thinking.
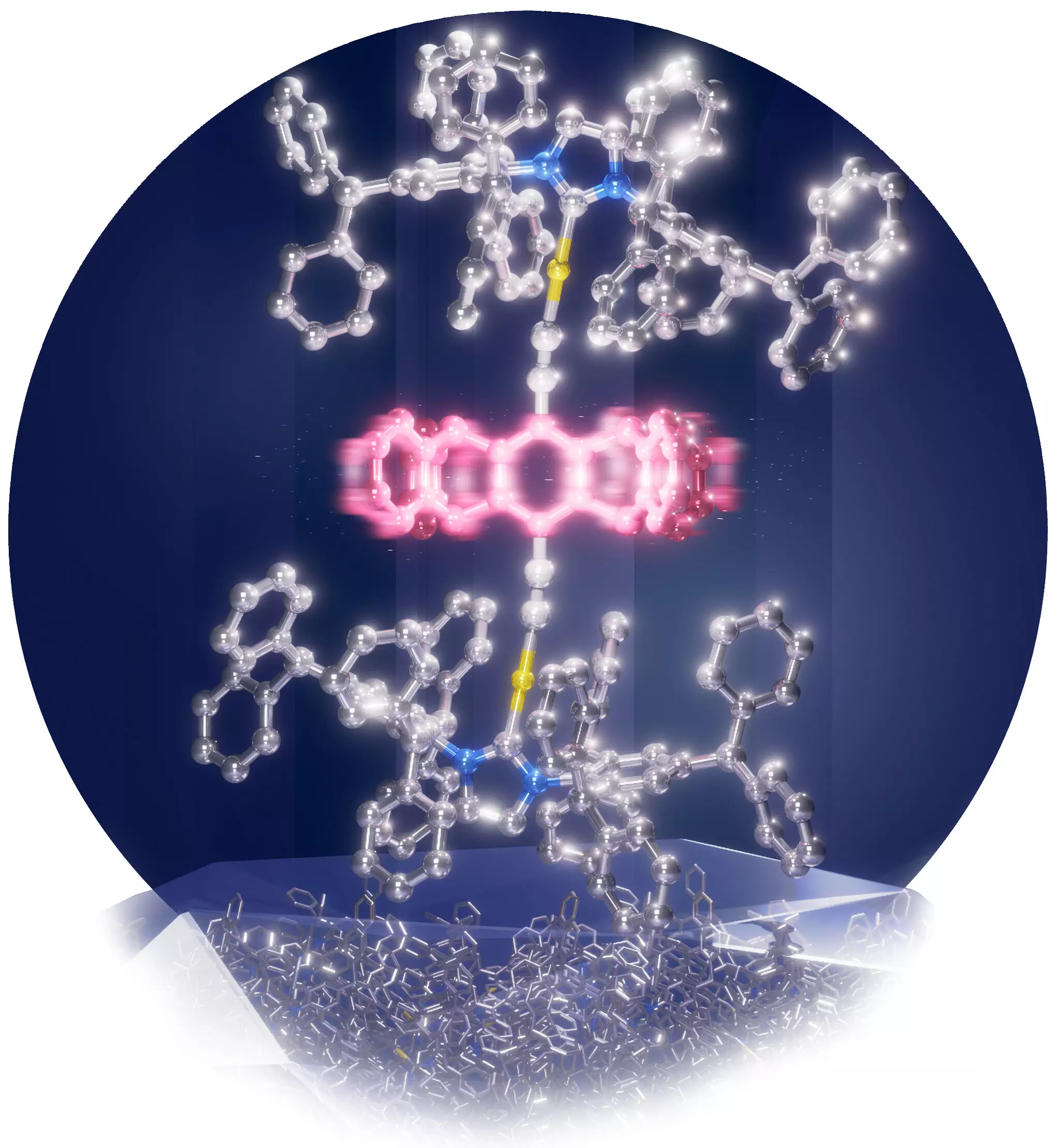
A team led by Associate Professor Mingoo Jin from the Institute for Chemical Reaction Design and Discovery (WPI-ICReDD) at Hokkaido University has achieved a significant breakthrough in the field. Their study, published in the journal Angewandte Chemie International Edition, describes the development of a molecular rotor that operates in the solid-state. This molecular rotor consists of a central rotating molecule connected to stationary stator molecules. The novel aspect of this study lies in the size of the rotor molecule, which is nearly 40% larger in diameter compared to previous rotors in the solid-state.
Enabling rotation in such a large molecule required creating sufficient free space within the solid. To achieve this, the research team synthesized concave, umbrella-like metal complexes that could shield the rotor molecule from unwanted interactions with other molecules in the crystal. By attaching a large, bulky molecule to the metal atom of the stator, they successfully created the necessary space to accommodate the giant rotor. This innovative approach was inspired by the protective nature of an egg, which utilizes a circular hardcover to create a large space and safeguard its contents.
The experimental and simulated nuclear magnetic resonance spectra of the crystal provided fascinating insights about the giant molecular rotor. It was discovered that the rotor rotates in 90-degree intervals at a frequency ranging from 100 to 400 kHz. This groundbreaking discovery expands the realm of possibilities for molecular motion in the solid-state, paving the way for further exploration in the development of amphidynamic crystals. Moreover, it opens up a path towards the creation of new functional materials with unique and desirable properties.
The utilization of pentiptycene rotators in this study, which possess several pocket sites, highlights the potential for exploring additional avenues in the development of new functional materials. The knowledge and techniques gained from this research contribute to our understanding of molecular motion in solids and inspire future endeavors. The ability to incorporate moving parts into solid materials has far-reaching implications in various disciplines, including materials science, chemistry, and nanotechnology.
The exploration of moving parts within solid materials revolutionizes our understanding of the properties and possibilities of these materials. The remarkable achievement of a functional molecular rotor in the solid-state, featuring a large, 40% larger in diameter than previous rotors, paves the way for the development of amphidynamic crystals and exciting new functional materials. This breakthrough exemplifies the power of creative thinking and innovative solutions in the field of science and engineering. As we continue to push the boundaries of what is possible, the future holds incredible potential for the development of materials with unprecedented properties and applications.


Leave a Reply