Hydrogen (H2) has long been recognized as a promising fuel for reducing greenhouse gases, particularly when produced through the splitting of water molecules (H2O) using renewable energy sources. Despite the apparent simplicity of this process, the chemistry behind breaking water into hydrogen and oxygen is extraordinarily complex. This complexity arises from the need for catalysts to facilitate two simultaneous electrochemical reactions essential for the water-splitting process.
Recently, a team of scientists at the U.S. Department of Energy’s Brookhaven National Laboratory and Columbia University announced a significant advancement in catalyst technology. They have successfully developed an innovative catalyst that significantly enhances the efficiency of the oxygen evolution reaction, which is the more challenging part of the water-splitting process.
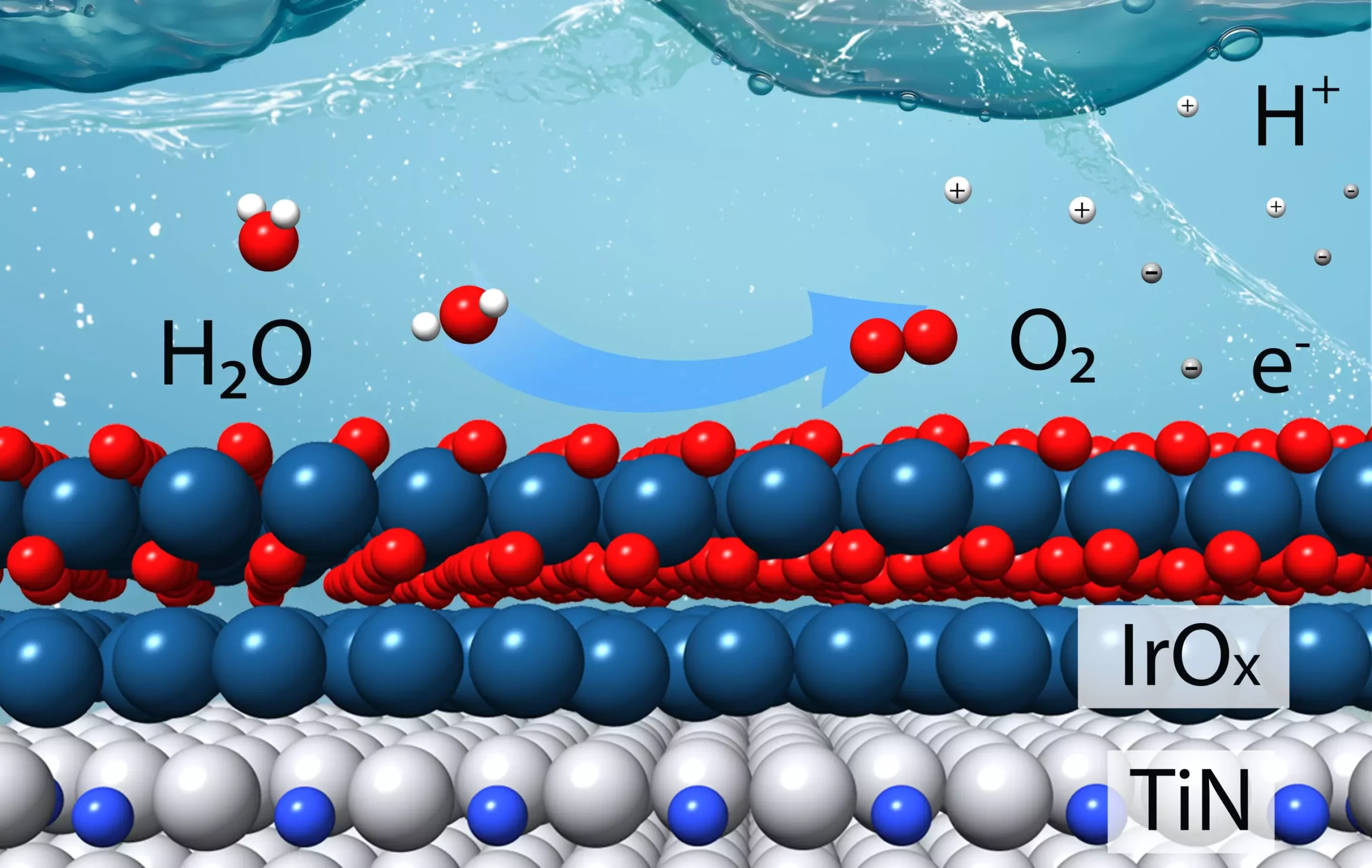
The catalyst, designed “from the bottom up” based on theoretical calculations, aims to minimize the reliance on iridium – a costly metal commonly used as a catalytic material. Through thorough testing and validation in laboratory experiments, the team confirmed that their catalyst outperformed the current state-of-the-art iridium catalyst by a significant margin.
The primary motivation behind this research endeavor stems from the limited availability and high cost of iridium, making it impractical for large-scale hydrogen production. By utilizing earth-abundant materials, such as titanium, as the core for the catalyst, and subsequently coating it with a thin layer of iridium, the team managed to achieve both stability and efficiency in acidic reaction conditions.
The fundamental breakthrough in catalyst design involved utilizing theoretical calculations to determine the ideal number of iridium layers required to drive the oxygen evolution reaction effectively. Such calculations provided crucial insights into the stability and activity of the catalyst under challenging conditions, paving the way for experimental validation and real-world application.
Extensive characterization studies, involving thin films and powdered samples of the catalyst, revealed the intricate interactions between iridium and titanium atoms under reaction conditions. These studies not only confirmed the predicted outcomes but also shed light on the underlying mechanisms that govern the catalyst’s performance.
By precisely controlling the layers in thin films and optimizing the composition of powdered samples, the scientists were able to fine-tune the catalyst’s activity and stability. The charge transfer between titanium and iridium surfaces played a pivotal role in enhancing the binding of reaction intermediates, thereby optimizing the overall catalytic performance.
While the development of this novel catalyst marks a significant milestone in green hydrogen production, the scientists acknowledge the need for further advancements to scale up production and improve the consistency of catalyst powders. By providing guidelines for industrial chemists to create core-shell structures with a uniform iridium layer, the research opens up new possibilities for lowering the cost of water splitting and driving large-scale green hydrogen production.
In essence, the journey from theoretical calculations to experimental validation has brought us closer to a sustainable future powered by green hydrogen. The relentless pursuit of innovation and the collaborative efforts of scientists have the potential to revolutionize the way we produce, store, and utilize clean energy sources for generations to come.


Leave a Reply