The growing crisis of climate change has drawn significant attention to the strategies capable of mitigating carbon dioxide (CO2) emissions, a major contributor to global warming. Among the various approaches under scrutiny, electrochemical reduction stands out due to its potential to convert this harmful gas into valuable fuels and chemicals. Recently, a collaborative effort by researchers from the U.S. Department of Energy’s Brookhaven National Laboratory, Yale University, and the University of North Carolina at Chapel Hill has unveiled a promising advancement in this domain. Published in the Journal of the American Chemical Society, their work demonstrates an extraordinary enhancement in catalytic efficiency that could reshape the landscape of CO2 reduction.
As industries continue to produce CO2 through electricity generation, transportation, and manufacturing, finding effective catalysts for its conversion remains a critical challenge. A catalyst accelerates the rate of chemical reactions but often requires substantial energy input, which poses economic barriers to large-scale implementation. Gerald Manbeck, a chemist from Brookhaven, emphasizes that while several materials display catalytic properties, many demand excessive energy for practical use, limiting their viability. The innovative catalyst identified by this research promises enhanced performance and lower energy requirements, presenting a new avenue for the future of catalyst design.
Introducing Rhenium-Based Catalysts
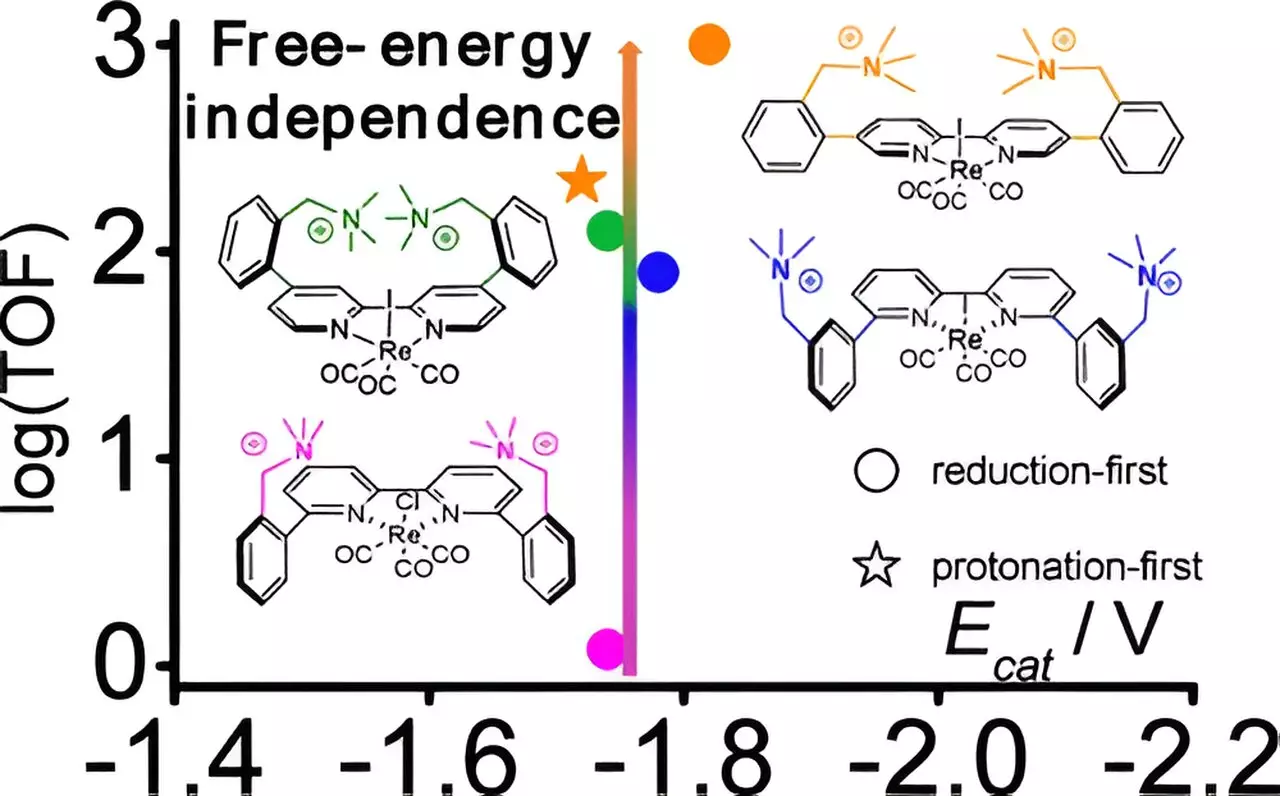
The research team focused on a known catalyst rooted in rhenium—a transition metal recognized for its catalytic prowess. Rhenium’s atomic structure, when supported by organic fragments, serves as the reaction center. However, the team sought to innovate further by modifying this existing framework. They introduced cations—positively charged molecules—strategically positioned at varying distances from the rhenium center. This meticulous adjustment proved to be a game changer. They discovered a specific spatial configuration that resulted in an astonishing 800-fold increase in catalytic activity, achieved without the necessity for significant extra electrical energy input.
Manbeck’s team relied on computational chemistry to unveil the intricacies that underlie their discovery. They identified that the carefully placed cations stabilize the latter stages of the catalytic process, facilitating a previously overlooked low-energy pathway in rhenium-based molecular catalysis. This revelation underscores the importance of optimizing catalyst geometry—a nuance that can lead to remarkable advancements in catalytic efficiency.
To substantiate their findings, the research group employed multiple sophisticated techniques. Cyclic voltammetry provided valuable measurements detailing energy characteristics and reaction kinetics. Simultaneously, infrared spectroelectrochemistry revealed critical structural alterations during the reaction, enabling the team to track changes near the catalyst’s interface. The use of a novel apparatus, designed by team members, allowed for unprecedented sensitivity in monitoring these chemical transformations closely, marking a significant stride in experimental capabilities.
The implications of this research extend beyond merely improving catalytic efficiency; they pave the way for future explorations into integrating light-absorbing materials into their catalytic system. The prospects of harnessing sunlight through semiconductors, such as silicon, illustrate a powerful synergy in reducing reliance on external electrical energy. This promising avenue aligns with the mission of CHASE (Center for Hybrid Approaches in Sustainability and Energy), aiming to develop photoelectrodes that can harness solar energy to catalyze CO2 reduction in tandem with water.
The advancements reported by this group exemplify a critical intersection of chemistry, materials science, and sustainability. If scalable production of their catalyst is achievable, it could significantly contribute to the global effort to reduce atmospheric CO2 levels while generating renewable fuels. The integration of light energy with electrochemical processes has the potential to radically transform energy systems, turning our focus toward more sustainable and green methodologies.
This research also highlights the broader importance of interdisciplinary collaboration in addressing climate change. By merging knowledge from various fields, teams can unlock breakthroughs that single-discipline approaches cannot achieve. As these scientists continue to delve deeper into the realms of catalysis, the pursuit of innovative solutions to combat CO2 emissions appears more promising than ever. Ultimately, the work done in this direction not only contributes to combating climate change but may also reshape future energy landscape, making it a vital area of research in the context of global sustainability efforts.

Leave a Reply