The University of Michigan researchers have made a groundbreaking discovery in the field of renewable energy by developing a catalyst material called cobalt phthalocyanine. This catalyst has the ability to convert carbon dioxide, a notorious contributor to climate change, into renewable fuels like methanol. This development has the potential to revolutionize the way we view carbon emissions and clean energy production.
The process involves a series of steps where carbon dioxide is first converted into carbon monoxide and then further transformed into methanol using cobalt phthalocyanine as a catalyst. This approach offers a sustainable solution to reduce greenhouse gas emissions while simultaneously creating a pathway to generate clean energy. The idea of converting CO2 into useful fuels like methanol has been a long-standing challenge in the scientific community. Methanol, if produced efficiently, could be used as a clean energy source for vehicles, thus reducing our reliance on fossil fuels.
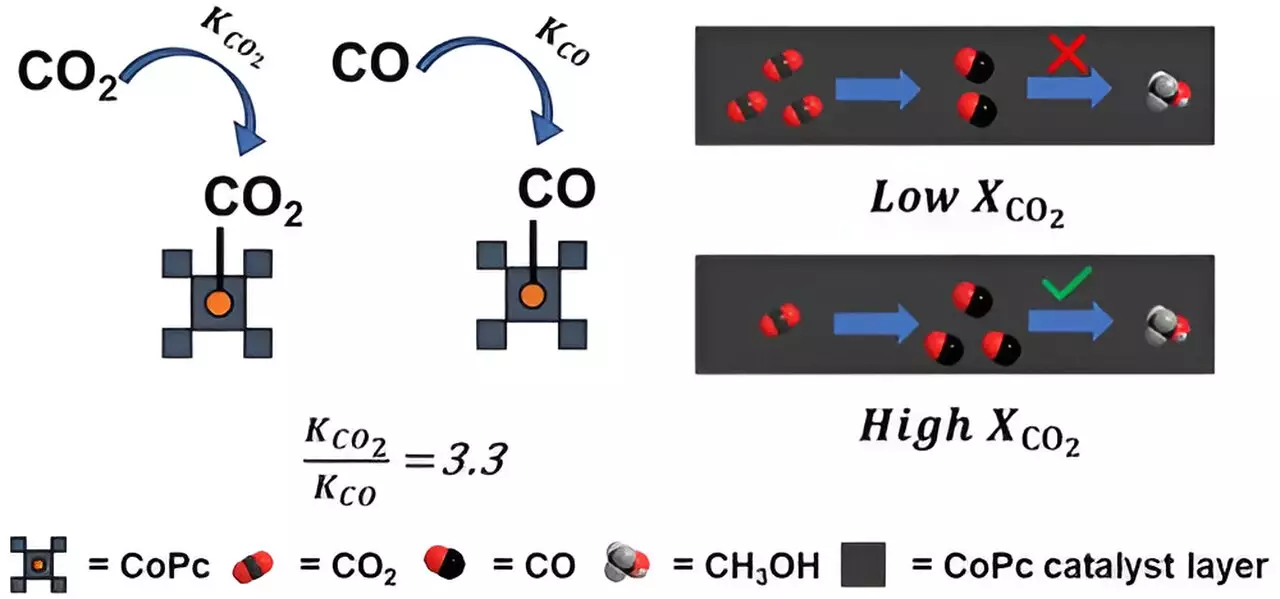
While the conversion of CO2 to methanol has been achieved on an industrial scale, doing so through electrochemical processes presents a formidable challenge. The researchers discovered that cobalt phthalocyanine binds to CO2 molecules much more strongly than to CO molecules, leading to inefficiencies in the conversion process. This results in the displacement of CO by another CO2 molecule before it can be fully converted to methanol.
To address this issue, the researchers propose redesigning the cobalt phthalocyanine catalyst to enhance its interaction with CO and reduce its binding affinity to CO2. By modifying the catalyst’s electron interactions with CO2 and CO molecules, they aim to create a more efficient system for converting CO2 waste into methanol fuel on a large scale.
The development of cobalt phthalocyanine as a catalyst for converting carbon dioxide into renewable fuels marks a significant advancement in the field of sustainable energy production. By overcoming the challenges associated with the binding affinity of the catalyst, researchers have opened up new possibilities for mitigating greenhouse gas emissions and generating clean energy. This research paves the way for a greener future where CO2 waste can be repurposed into valuable resources, making strides towards a more environmentally friendly world.


Leave a Reply