Ethylene is widely regarded as the most crucial chemical in the petrochemical industry, serving as a vital raw material for numerous everyday products. Currently, the production of ethylene relies on a resource-intensive process known as steam cracking, which emits large amounts of carbon dioxide into the atmosphere. However, a greener alternative called oxidative coupling of methane (OCM) has emerged. While previous attempts at OCM have fallen short in terms of ethylene production yield, researchers from North Carolina State University (NCSU) and Lehigh University have made a significant breakthrough, achieving a single-pass yield of over 30%. This achievement is a step closer to making OCM a commercially viable option for ethylene production.
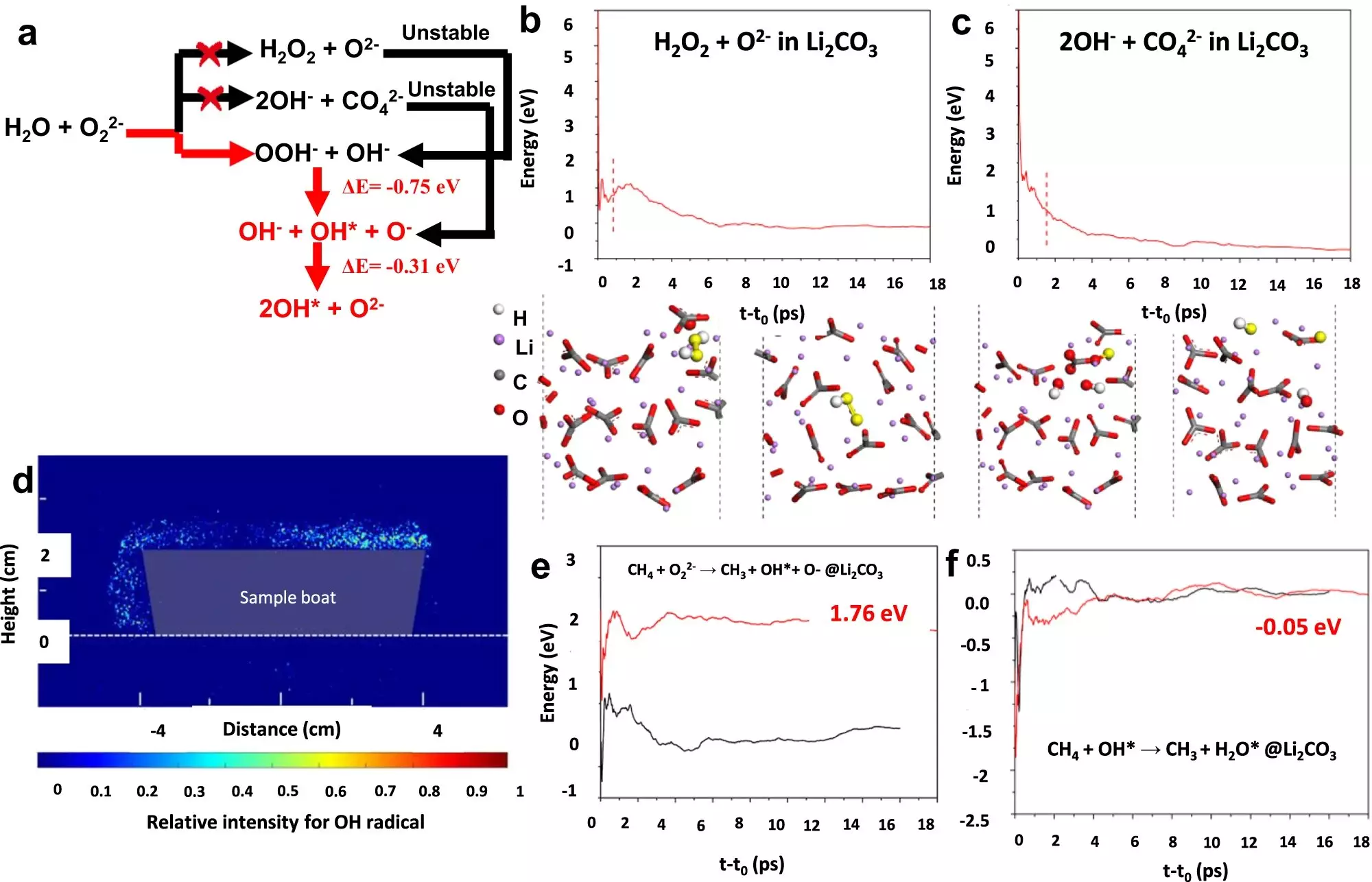
Led by Fanxing Li, the research team developed a class of core-shell Li2CO3-coated mixed rare earth oxides as catalysts for OCM, utilizing a chemical looping scheme. This innovative approach involves sequentially introducing methane and oxygen to the catalyst, ensuring continuous reoxidation and replenishment of the catalyst’s oxygen supply. Through this method, the researchers achieved a single-pass yield of up to 30.6%.
The team at Lehigh University, under the leadership of Israel Wachs, played a crucial role in characterizing the catalyst. They specialize in in situ surface characterization, enabling them to analyze catalyst surfaces while the reaction is ongoing. This comprehensive understanding of catalyst transformations during the catalytic reaction is key to determining the catalyst’s effectiveness. The catalyst used in this study consists of a mixed oxide core covered by lithium carbonate. The interaction between the core and shell during chemical looping contributes to the high ethylene production yield.
The successful development of a catalyst with a yield exceeding 30% brings the potential for cost-effective and energy-efficient ethylene production using OCM closer to reality. In addition to reducing emissions, OCM offers the advantage of utilizing methane as a feedstock, which can be sourced from natural gas, biogas, or even electrochemically reduced carbon dioxide. Ethylene, which can be derived from methane through OCM, is a crucial building block for a wide range of products consumed globally.
Moving forward, researchers aim to assess the suitability of this catalyst for industrial-scale production while striving to further enhance the yield. The ultimate goal is to improve upon a method that has remained unfulfilled since the 1980s. The complex dynamics involved in the OCM process present a unique challenge, but the remarkable discoveries made during this study show promise for optimizing ethylene production. Each advancement brings us closer to environmentally friendly and sustainable alternatives for meeting the petrochemical industry’s demands.
The recent breakthrough in OCM catalyst development is a significant milestone in the journey towards greener ethylene production. By surpassing a yield of 30%, researchers at NCSU and Lehigh University have demonstrated the potential for OCM to become an economically viable alternative to steam cracking. This achievement not only reduces carbon emissions but also utilizes methane, a readily available resource, as the primary feedstock. As the catalyst continues to undergo further refinements, the dream of cost-effective, energy-efficient, and environmentally friendly ethylene production through OCM becomes increasingly attainable. This breakthrough serves as a testament to the ingenuity and dedication of scientists working towards a sustainable future.


Leave a Reply