As the world moves towards renewable energy, there is a need for new technologies for storing and distributing energy to homes and electric vehicles. The current standard for energy storage has been the lithium-ion battery containing liquid electrolytes. However, this solution is far from ideal due to its low efficiency and the liquid electrolyte’s propensity to occasionally catch fire and explode. Many public and private research labs are spending a lot of time and money to develop alternative solid-state batteries out of various materials. This approach offers a much safer and more stable device with a higher energy density than lithium-ion batteries, at least in theory.
The Promise of Argyrodites
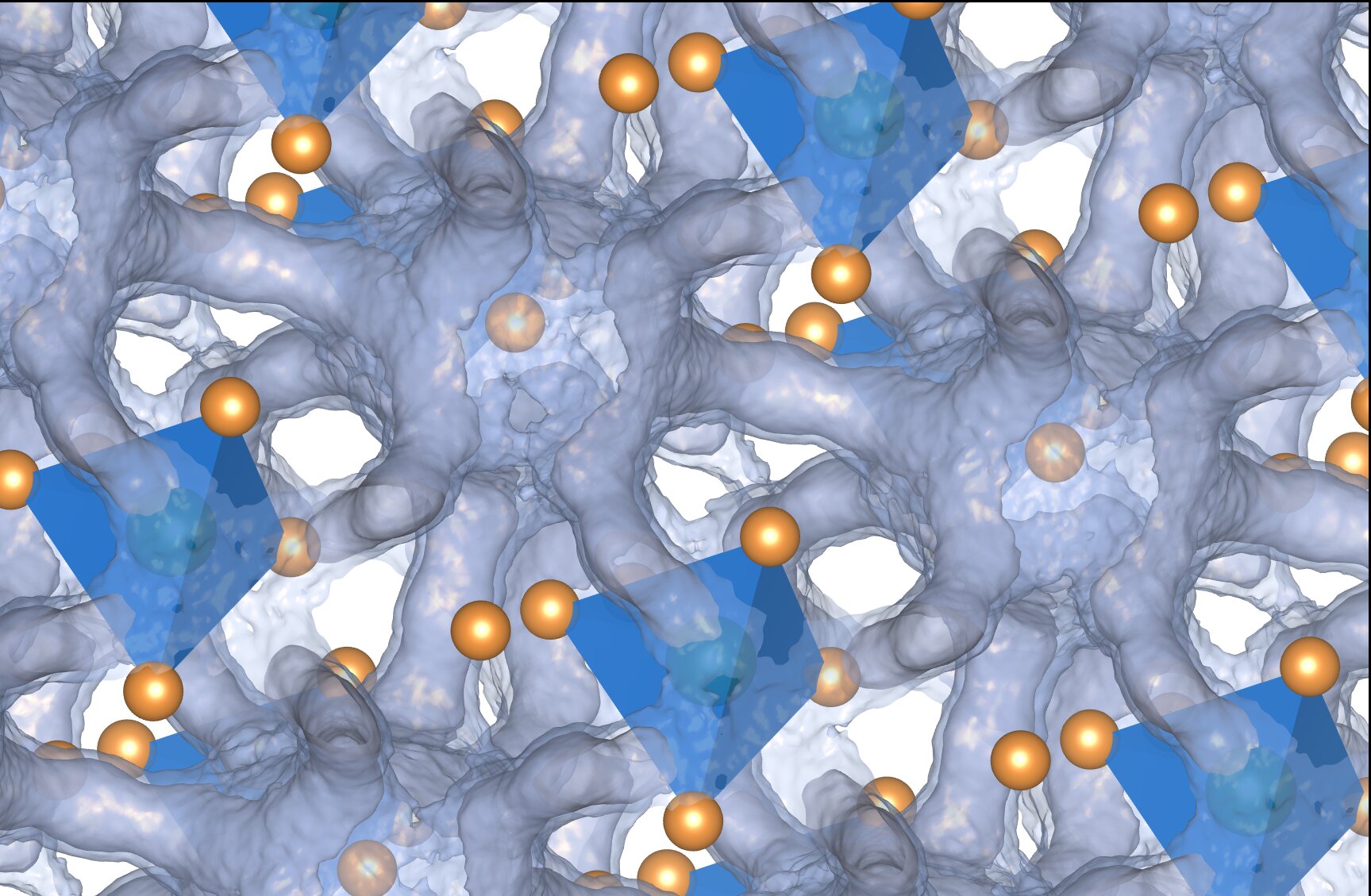
One of the leading contenders for solid-state batteries relies on a class of compounds called argyrodites. These compounds are built from specific, stable crystalline frameworks made of two elements with a third free to move about the chemical structure. While some recipes such as silver, germanium, and sulfur are naturally occurring, the general framework is flexible enough for researchers to create a wide array of combinations. If engineered correctly, this approach offers a much safer and more stable device with a higher energy density.
Discovering the Atomic Mechanisms for Argyrodites
A team of researchers at Duke University and their collaborators have uncovered the atomic mechanisms that make argyrodites attractive candidates for both solid-state battery electrolytes and thermoelectric energy converters. The researchers looked at one promising candidate made of silver, tin, and selenium (Ag8SnSe6). Using a combination of neutrons and X-rays, the researchers bounced these extremely fast-moving particles off atoms within samples of Ag8SnSe6 to reveal its molecular behavior in real-time. The researchers used a machine learning approach to make sense of the data and created a computational model to match the observations using first-principles quantum mechanical simulations.
The Dual Nature of Argyrodites
The results showed that while the tin and selenium atoms created a relatively stable scaffolding, it was far from static. The crystalline structure constantly flexes to create windows and channels for the charged silver ions to move freely through the material. The system is like the tin and selenium lattices remain solid while the silver is in an almost liquid-like state. The duality of a material living between both a liquid and solid state is what the researchers found most surprising.
The results and the approach combining advanced experimental spectroscopy with machine learning should help researchers make faster progress towards replacing lithium-ion batteries in many crucial applications. According to the researchers, this study is just one of a suite of projects aimed at a variety of promising argyrodite compounds comprising different recipes. Many of these materials offer very fast conduction for batteries while being good heat insulators for thermoelectric converters, so the researchers are systematically looking at the entire family of compounds. This study serves to benchmark the machine learning approach that has enabled tremendous advances in the ability to simulate these materials in only a couple of years. The researchers believe that this will allow them to quickly simulate new compounds virtually to find the best recipes these compounds have to offer.


Leave a Reply