A team of Chinese scientists from the University of Science and Technology of China (USTC) has developed a new catalyst for hydrogen evolution reaction (HER). The researchers have created a general strategy for the synthesis of ten single-atom doped CoSe2-DETA (DETA = diethylenetriamine) nanobelts. The team has optimized the hydrogen evolution reaction (HER) activity of the products by adjusting the doped single-atom ions and modulating the electronic structure. The research has been published in the journal Nature Communications.
New Catalyst for Hydrogen Evolution Reaction
Hydrogen is a renewable and clean energy source that could replace fossil fuels. Producing “green hydrogen” through HER could be a wise way to embrace a hydrogen-supporting society. Currently, platinum (Pt) and Pt-based materials are the only commercially available HER electrocatalysts because of their unique electronic structure. However, their high price and scarcity prevent large-scale commercial applications. Therefore, it is urgent to design low-cost HER electrocatalysts with high catalytic activity and stability.
Optimizing the electronic structure of catalysts can improve the intrinsic properties of the material. Among them, ion doping is an effective method to optimize HER performance by introducing heteroatoms into the material lattice. This strategy changes the local coordination and modulates the electronic structure, which improves catalytic performance.
New Synthetic Methodology for HER Catalysts
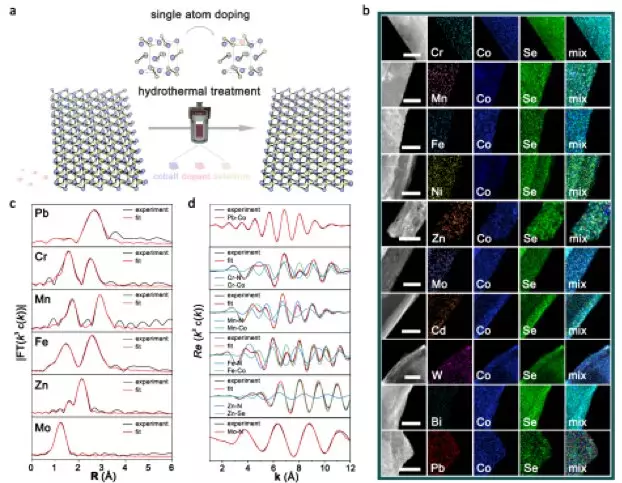
The team of Chinese scientists has developed a general single-atom doping strategy for the preparation of ten single-atom doping products by replacing Co with different cations. Assisted by different doping atomic systems, the local coordination of the products can be modulated to realize the controllable adjustment of electronic structures and HER behaviors over a wide range.
The experimental results showed that the catalytic activity of the optimal doped product is similar to that of commercial Pt/C (40 wt%). The overpotential required to reach a current density of 10 mA/cm2 is only 74 mV, with a Tafel slope of 42 mV/dec calculated from the polarization curve. In addition, accelerated voltammograms (CVs) cycling tests proved the long-term stability of the catalysts.
The activity of the catalysts remains almost constant over 1000 CVs cycles, and the product is able to operate stably under a constant current density of 10 mA/cm2 for 20 hours. The synchrotron spectroscopy data showed that the coordination environment of the Co atoms (ratio of Co-N bonds to Co-Se bonds) in the product varies with different dopant atoms.
Additionally, a volcano-shaped relationship between the coordination environment and the HER activity of the product was summarized, demonstrating the structure-property relationship of doped CoSe2. This work provides a novel method for the design and synthesis of high-efficiency catalysts, and the optimal products obtained are expected to substitute the commercial Pt/C catalysts and become ideal electrode materials.
The team of Chinese scientists has developed a new catalyst for HER by creating a general strategy for the synthesis of ten single-atom doped CoSe2-DETA nanobelts. This new catalyst has high catalytic activity, stability and is expected to replace the commercial Pt/C catalysts. The team’s work provides a novel method for the design and synthesis of high-efficiency catalysts that could help to embrace a hydrogen-supporting society.


Leave a Reply