Organic semiconductors have captured the attention of researchers due to their flexibility, light weight, and potential for unique electronic functionalities. Unlike conventional rigid semiconductors, organic molecules offer greater flexibility in molecular design, opening up new possibilities for the development of semiconductor devices. One such molecule that has recently garnered interest is the synthetic derivative of the natural dye indigo, known as 3,3-dihydroxy-2,2-biindan-1,1-dione (BIT). In a groundbreaking study published in Chemical Science, chemists at RIKEN have developed a room-temperature method for synthesizing BIT derivatives, paving the way for advancements in the field of organic semiconductors.
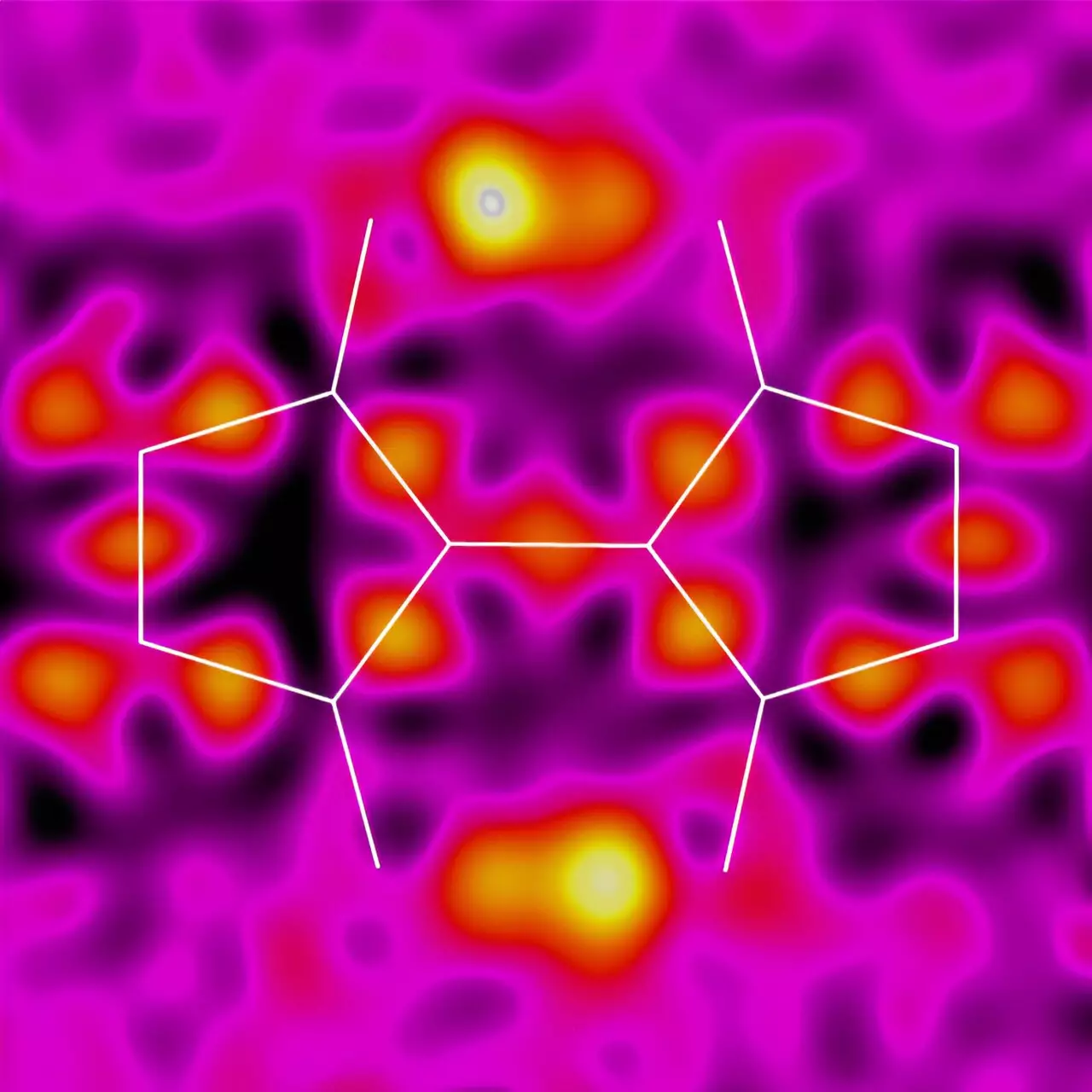
Proton-coupled electron transfer (PCET) plays a crucial role in efficient electron transfer in biological systems. However, incorporating PCET into solid-state materials has proven to be a challenge. Led by Keisuke Tajima, the research team at RIKEN sought to investigate whether BIT and its derivatives could display PCET and exhibit unique dynamic properties. The team observed unusual rearrangements in the structures of BIT and its derivatives involving double-proton transfer, indicating the potential for these materials to possess electronic functional properties.
Improved Synthesis Method
One of the key hurdles in exploring the properties of BIT derivatives was the harsh conditions required for their synthesis, limiting the range of possible derivatives. However, the RIKEN team successfully developed a room-temperature approach that enabled the synthesis of several BIT derivatives under milder conditions. This breakthrough not only broadened the range of derivatives that could be made but also made it possible to investigate the properties of these materials in greater detail.
The team’s next challenge was to prove that the protons in BIT undergo proton transfer between molecules in the solid state. Collaborating with experts in X-ray crystallography and solid-state nuclear magnetic resonance (NMR), the research team was able to demonstrate that the two protons in BIT do rapidly exchange their positions. Calculations further suggested that proton transfer is coupled with charge transport, opening up a realm of possibilities for enhanced electron transfer in these materials. However, further experimental confirmation is required to fully establish this coupling.
Future Directions
The discovery of the room-temperature synthesis method for BIT derivatives and the demonstration of proton-coupled electron transfer in solid-state materials lay the foundation for future advancements in organic semiconductors. The presence of a proton in BIT derivatives could potentially enhance charge transport, providing exciting avenues for further exploration. By harnessing the flexibility in molecular design offered by organic semiconductors, this research paves the way for the development of electronic devices with improved performance, such as light-responsive gadgets and stretchy biomedical sensors.
The study conducted by the chemists at RIKEN showcases the potential of organic semiconductors for revolutionizing the field of electronics. Starting with a simple question about the possibility of protons and electrons moving in harmony in the solid state, the researchers have not only discovered a method for synthesizing BIT derivatives under milder conditions but also established the existence of proton-coupled electron transfer in these materials. As the research progresses, these findings have the potential to lead to the development of a new generation of flexible, lightweight, and highly functional electronic devices.


Leave a Reply