A team of researchers from the University of Michigan has made a groundbreaking discovery in the field of plant biochemistry. In a study published in the journal Nature Chemical Biology, the researchers have identified a new mechanism by which plants generate cyclic peptides. These molecules have the potential to be used in pharmaceuticals as they can bind to challenging drug targets. Led by Lisa Mydy and Roland Kersten from the College of Pharmacy, the team’s findings open up new possibilities in drug research and development.
Mydy and her colleagues focused their research on the biosynthesis of macrocyclic peptides found in plants, which have been recognized for their therapeutic drug potential. During their investigation, they made a remarkable discovery. They identified a previously unknown protein fold that exhibits a unique mechanism for forming cyclic peptides. Mydy described it as “a new biochemistry that we have not seen before.” This exceptional protein fold has the capacity to generate cyclic peptides, including one that shows promise as an anti-cancer drug.
A Protein Shrouded in Mystery
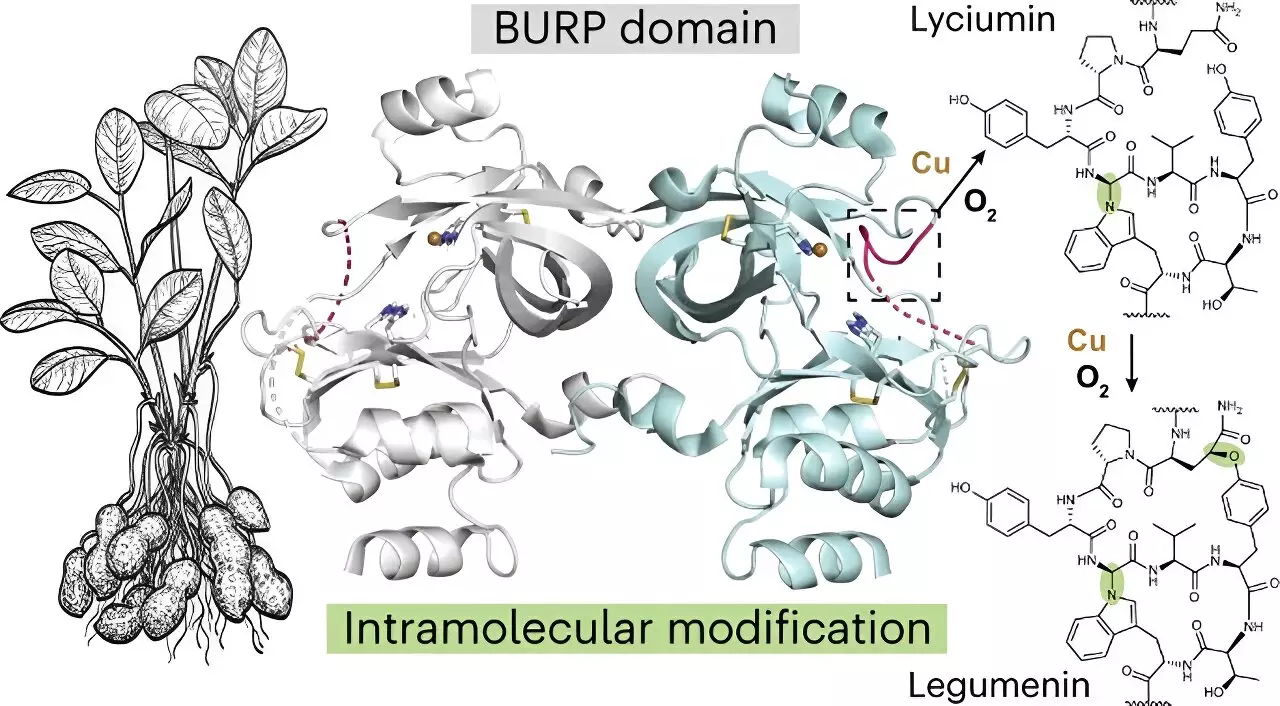
Prior to this study, there was little information available about the protein called AhyBURP, which plays a crucial role in peptide cyclization. Mydy explained that the only clue they had about its function was that it required copper to cyclize a peptide. To unravel its mysteries, the researchers utilized X-ray crystallography to study the protein’s structures. They also utilized the Advanced Photon Source at Argonne National Laboratory, which provided valuable insights into AhyBURP’s unique use of copper and oxygen.
One of the most intriguing aspects of AhyBURP is its ability to perform cyclization chemistry within the same protein. Typically, other cyclic peptides require the involvement of another enzyme to accomplish this process. Mydy elaborated on this phenomenon, stating that AhyBURP can achieve cyclization on its own. Moreover, the researchers observed that AhyBURP does not attach oxygen to the peptide like other copper-dependent proteins. These extraordinary properties make it the first known instance of this type of chemistry happening within a protein.
Potential Drug Development
The discovery of this new protein and its unique properties is a significant development in the field of drug research. The team at the University of Michigan has been diligently working on the Natural Product Discovery Initiative, aiming to identify plant-based chemicals with drug potential. Cyclic peptides are of particular interest due to their enhanced structure and stability, making them viable candidates for pharmaceutical applications. The researchers are hopeful that their findings will pave the way for the development of new drugs, especially to combat diseases like cancer.
The potential of cyclic peptides as therapeutic agents is immense. Many drugs, both synthetic and derived from living organisms, rely on cyclic structures to bind to specific drug targets and remain intact in a patient’s body for an extended period. The natural world has offered numerous biochemical solutions for producing cyclic molecules, and the discovery of AhyBURP adds to this repertoire. Additionally, Kersten’s lab has already identified other compounds produced by the same protein family that exhibit suppressing effects on lung cancer cells. This finding fuels optimism that this breakthrough will contribute to the development of effective anti-cancer agents.
With knowledge of the protein’s structure, the researchers can now delve deeper into understanding the chemical reactions between the peptide, copper, and oxygen that lead to the formation of cyclic peptides. Mydy, a structural biologist and enzymologist, expressed her excitement about the challenges posed by this puzzle. Unraveling the underlying mechanisms of this process holds the key to unlocking the full potential of AhyBURP and its implications for drug development.
The University of Michigan researchers’ discovery of a new plant biochemistry and the identification of a novel protein fold hold tremendous promise in the field of drug development. By shedding light on the mechanism by which plants generate cyclic peptides, the study opens up doors for the creation of innovative pharmaceuticals. The extraordinary properties of AhyBURP and its ability to self-cyclize within the same protein make it a significant breakthrough. This newfound knowledge will undoubtedly lead to further research and exploration, paving the way for the development of new drugs to combat various diseases and improve human health.


Leave a Reply