The utilization of the greenhouse gas CO2 as a chemical raw material has the potential to not only decrease emissions but also reduce the consumption of fossil feedstocks. One way to achieve this is through the electrocatalytic production of ethylene, a crucial chemical raw material, from CO2. Ethylene serves as a starting material for the production of various products, including polyethylene and plastics. Traditionally, ethylene is generated through high-energy cracking and rectification of fossil feedstocks. However, electrochemical conversion of CO2 to ethylene presents a promising alternative route that could significantly decrease CO2 emissions while conserving energy and fossil resources.
The electrochemical conversion of CO2 into ethylene poses several challenges due to the stability of CO2 and the complexity of forming the necessary bond between two carbon atoms. Prior to this study, copper catalysts were predominantly used to achieve this conversion. However, the use of metals in catalysis can be costly and environmentally problematic. In pursuit of a more sustainable approach, a team of researchers led by Chengtao Gong and Fu-Sheng Ke at Wuhan University, China, developed a metal-free electrocatalyst for the conversion of CO2 to ethylene.
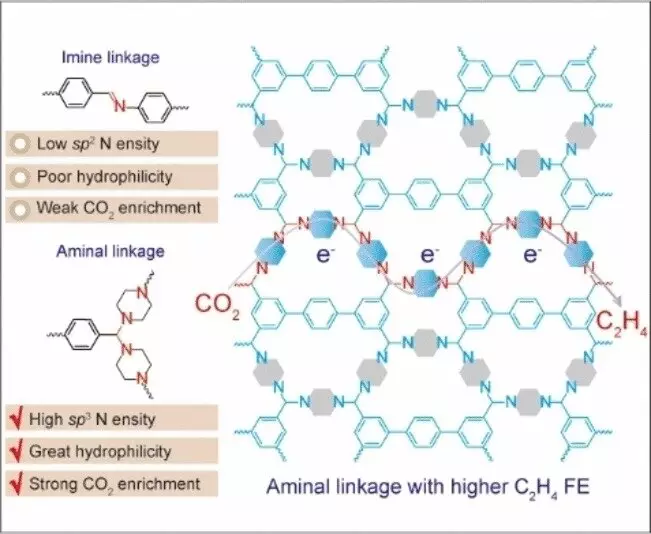
The catalyst devised by the team is based on a nitrogen-containing covalent organic framework (COF). COFs represent a new class of porous, crystalline, organic materials with defined topology. Unlike metal-organic frameworks (MOFs), COFs do not require metal ions for structural integrity. The nitrogen atoms within the COF possess a unique electron configuration (sp3 hybridization) that serves as catalytically active centers. These sp3 nitrogen centers facilitate the binding of building blocks through an aminal link, resulting in the formation of frameworks through ring closures.
The success of the developed COF as an electrocatalyst for producing ethylene stems from the high density of active sp3-nitrogen centers. These centers efficiently capture CO2 and transfer electrons, leading to a concentration of excited intermediates capable of undergoing C–C coupling. In contrast, COFs with sp2 nitrogen centers failed to produce ethylene, highlighting the significance of the proper electron configuration for the electrochemical reduction of CO2 to ethylene.
The innovative use of a metal-free nitrogen-containing COF as an electrocatalyst for the conversion of CO2 to ethylene represents a significant advancement in sustainable chemical production. By leveraging the unique properties of COFs, such as their tunable pore sizes and catalytic activity, researchers are paving the way for more environmentally friendly and energy-efficient processes in the chemical industry. This breakthrough not only demonstrates the potential of using CO2 as a valuable raw material but also underscores the importance of considering electron configuration in the design of efficient catalysts for complex transformations.


Leave a Reply