The development of catalysts for hydrogen production through water electrolysis has been a challenging endeavor that researchers have been actively pursuing. However, the reliance on precious metal catalysts such as iridium has proven to be economically unfeasible, limiting the scalability of this environmentally friendly technology. In order to overcome this challenge, scientists have been exploring the potential of metal alloys as catalysts, specifically iridium, ruthenium, and osmium, which are the primary catalysts under scrutiny in the field of water electrolysis catalysis research.
Professor Yong-Tae Kim and Kyu-Su Kim from Pohang University of Science and Technology embarked on a research project to develop catalysts using a combination of iridium and ruthenium. By leveraging the excellent attributes of both metals, they aimed to enhance the activity and stability of the catalysts. Their efforts proved to be successful as the catalysts exhibited improvements in both activity and stability.
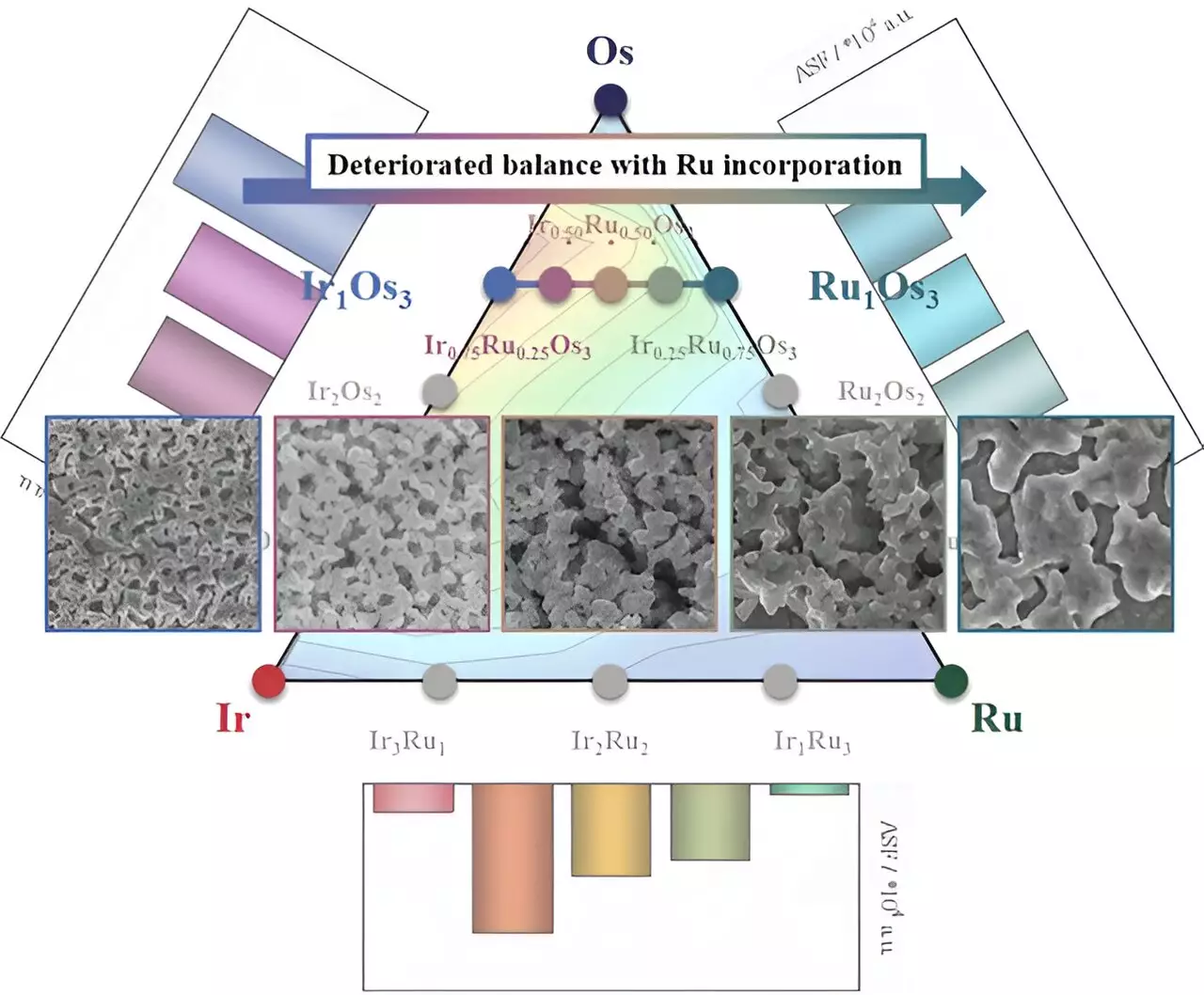
In addition to iridium and ruthenium, the researchers also explored the incorporation of osmium in the catalysts. Osmium, known for readily dissolving under various electrochemical conditions, offered the potential for the formation of nanostructures with an expanded electrochemical active surface area, thereby enhancing the geometrical activity. Catalysts incorporating osmium demonstrated high activity and retained the advantageous properties of iridium and ruthenium.
While the inclusion of osmium showed promise in terms of activity enhancement, its dissolution had a detrimental effect on the structural integrity of iridium and ruthenium. The accelerated agglomeration and corrosion of nanostructures compromised the overall balance of catalytic performance. These findings highlight the complexity of catalyst design and the importance of carefully considering the impact of each component.
In light of their experimental results, the research team has put forth several suggestions for further catalyst research. Firstly, they emphasize the need for a metric that can evaluate both activity and stability simultaneously. The activity-stability factor, introduced by Kim’s research group in 2017, serves as a promising tool for achieving this goal. Additionally, the team stresses the importance of retaining superior catalyst properties even after the formation of nanostructures to maximize the electrochemical active surface area. This requires careful selection of candidate materials that can effectively synergize when alloyed with other metals.
While this study does not present any specific outcomes in terms of new catalyst developments, it provides essential considerations for catalyst design. The combination of iridium and ruthenium has proven to be a successful strategy for enhancing both activity and stability. However, the inclusion of osmium introduces additional complexities that must be carefully managed. With further research and development, it is hoped that catalysts for hydrogen production can be optimized, paving the way for a sustainable and economically viable water electrolysis technology.
Recent advancements in catalyst development for hydrogen production have provided valuable insights into the challenges and potential solutions in the field of water electrolysis catalysis research. By critically analyzing the research conducted by Professor Yong-Tae Kim and Kyu-Su Kim, it becomes apparent that while the combination of iridium and ruthenium shows promise, the inclusion of osmium introduces complexities that need to be addressed. The proposed avenues for further research, such as the activity-stability factor and careful material selection, offer a path towards enhancing the performance of catalysts for water electrolysis. By continually pushing the boundaries of catalyst design, scientists can contribute to the development of a sustainable and economically feasible hydrogen production technology.


Leave a Reply