Researchers at the University of Cincinnati have made significant progress in the conversion of carbon dioxide into valuable products. Led by Associate Professor Jingjie Wu, the team has discovered that by using a modified copper catalyst, the electrochemical conversion of carbon dioxide into ethylene can be enhanced. Ethylene is a vital component in the production of numerous products, including plastics and textiles. This breakthrough not only promises a more efficient process but also contributes to tackling climate change by removing carbon from the atmosphere.
The conventional method of producing ethylene through the steam-cracking process has long been associated with substantial carbon dioxide emissions. In contrast, Wu’s team emphasizes utilizing carbon dioxide as a feedstock and transitioning to green energy sources instead of relying solely on fossil fuels. This not only reduces carbon emissions but also allows for the effective recycling of carbon dioxide. The significance of this discovery lies in its potential to revolutionize the production of ethylene globally and align it with sustainable practices.
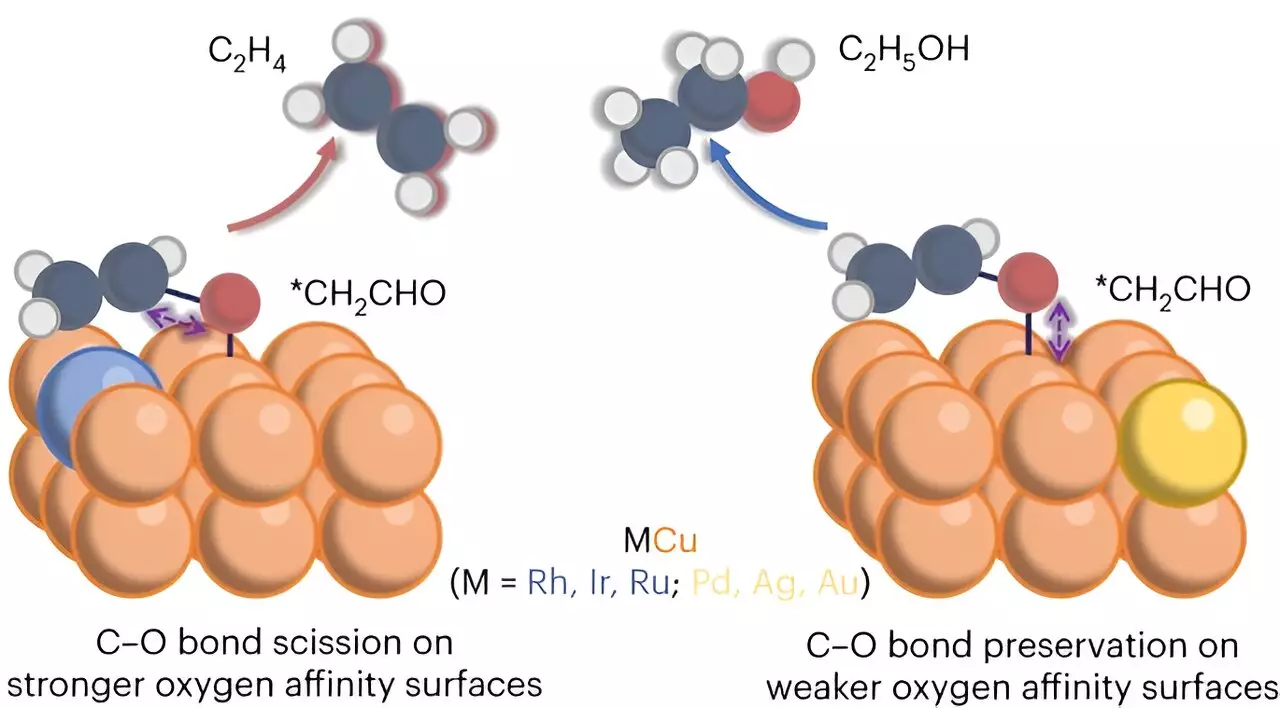
The research, published in Nature Chemical Engineering, explores the electrocatalytic conversion of carbon dioxide and its production of primary carbon products such as ethylene and ethanol. Through their experiments, the team found that the modified copper catalyst significantly increased the production of ethylene. This increased selectivity for ethylene by an impressive 50% represents a crucial step towards optimizing the conversion process. The ultimate goal is to streamline the process and focus on producing a single product instead of multiple byproducts.
Overcoming Challenges and Paving the Way Forward
While the team’s findings present a promising advancement, there are still obstacles to overcome for commercial deployment. One challenge lies in improving electrode stability as the formation of byproducts, such as potassium hydroxide, can affect the efficiency of the conversion system. To address this, the researchers are now focusing on enhancing stability and extending the operation period from its current limit of 1,000 hours to an ambitious goal of 100,000 hours. This would ensure long-term viability and practicality in industrial-scale applications.
The implications of these advancements extend beyond ethylene production and hold the potential to transform the chemical industry as a whole. Wu emphasizes the importance of decarbonizing chemical production by harnessing renewable electricity and sustainable feedstock. By integrating these new technologies, the industry can become more environmentally friendly and energy efficient, aligning with global efforts to combat climate change.
The research conducted at the University of Cincinnati represents a significant step forward in carbon dioxide conversion. By optimizing the electrochemical conversion process and utilizing a modified copper catalyst, the production of ethylene can be enhanced, reducing reliance on fossil fuels and minimizing carbon emissions. While there are challenges to be addressed for commercial deployment, these advancements pave the way for a greener and more sustainable future in the chemical industry. With continued progress and innovation, the vision of a carbon-neutral world becomes increasingly attainable.


Leave a Reply