The world is facing a pressing issue of greenhouse gas emissions, particularly carbon dioxide (CO2), contributing to global warming and climate change. However, what if these emissions could be transformed into valuable chemicals? A recent collaborative project between the U.S. Department of Energy’s (DOE) Argonne National Laboratory, Northern Illinois University, and Valparaiso University has brought forth a groundbreaking discovery – a low-cost, tin-based catalyst that can selectively convert CO2 into three widely produced chemicals: ethanol, acetic acid, and formic acid.
The Catalyst and Conversion Process
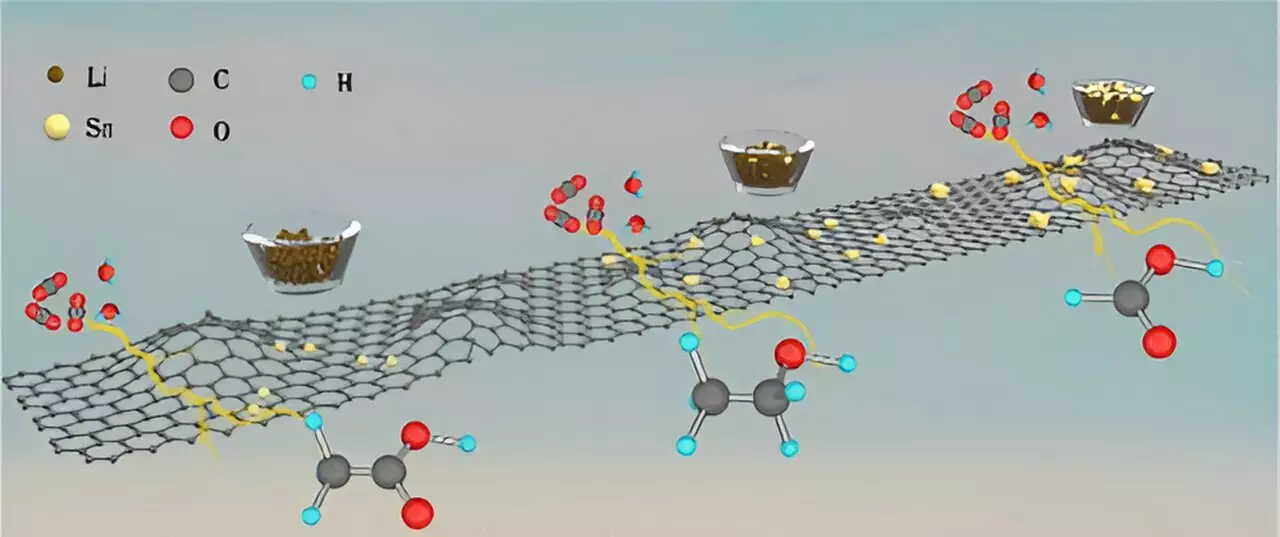
The catalysts developed by the research team consist of tin metal deposited over a carbon support. Through a process called electrocatalytic conversion, CO2 can be efficiently converted into ethanol, acetic acid, or formic acid by varying the size of tin used in the catalyst. This groundbreaking approach has shown a remarkable selectivity for each of the chemicals, with a percentage of 90% or higher. The ability to control the size of tin, from single atoms to larger nano-crystallites, allows for a precise conversion pathway to the desired chemical product.
Computational and experimental studies conducted by the team provided invaluable insights into the reaction mechanisms involved in the conversion process. An intriguing discovery was made regarding the significant impact of using deuterated water as opposed to ordinary water on the reaction path. This phenomenon, known as the kinetic isotope effect, had never been observed in CO2 conversion before. The research team’s findings shed light on the complexity of the conversion process and the potential for further optimization.
The success of the project was greatly facilitated by the utilization of two DOE Office of Science user facilities at Argonne – the Advanced Photon Source (APS) and the Center for Nanoscale Materials (CNM). With the advanced technology available at these facilities, the team was able to analyze the chemical and electronic structures of the tin-based catalysts with precision. The direct imaging of the arrangement of tin atoms, from single atoms to small clusters, provided crucial data for understanding the catalyst’s behavior.
Towards Sustainable Energy Integration
The ultimate goal of the research team is to integrate the newly developed catalysts into low-temperature electrolyzers powered by locally generated wind and solar energy. This innovative approach would allow for the production of desired chemicals at the source, reducing the need for extensive CO2 transport and storage. By leveraging renewable energy sources for the conversion process, the project aims to promote sustainability and reduce the environmental impact of industrial operations.
Implications for the Future
The discovery of the tin-based catalysts marks a significant step towards harnessing the potential of CO2 as a valuable resource for chemical production. With the ability to convert emissions into high-demand chemicals, the research has opened up new possibilities for sustainable industrial practices. By further refining the catalyst design and optimizing the conversion process, the project paves the way for a greener and more efficient approach to CO2 utilization.


Leave a Reply