Nature exhibits fascinating mechanisms for energy transformation, with photosynthesis serving as a primary example. Plants and certain bacteria convert light into chemical energy through this complex biological process. Similarly, in technological applications, solar panels utilize photovoltaic systems to convert sunlight into electrical energy. At the core of both phenomena lies the role of electronic motion, which facilitates charge transfer at the molecular level. Understanding the intricate processes that govern these transformations is crucial, especially as they unfold over extremely short timescales, often governed by quantum mechanics and molecular dynamics.
One of the most significant advancements in the study of electron dynamics in molecular systems is the ability to observe these phenomena with high temporal resolution. Measuring electron and charge transfer dynamics on the timescale of femtoseconds (10^-15 seconds) to attoseconds (10^-18 seconds) allows researchers to gain ground-breaking insights into the fundamental physical mechanisms at play. Such precision in observation not only reveals the intricate dance of electrons and nuclei but also aids in the engineering of chemical and structural attributes of molecules.
Recent developments have harnessed ultrashort ultraviolet pulses from high-order harmonic sources, alongside free electron laser facilities. These technologies enable scientists to initiate and observe molecular responses to photoionization processes, thereby delving deeper into ultrafast phenomena that were previously shrouded in mystery. Despite these advancements, however, a comprehensive understanding of the initial steps of electron and charge transfer remains elusive.
A noteworthy contribution to this field has emerged from a collaborative study among researchers at prominent institutions, including Politecnico di Milano and various universities in Madrid. Their work, published in *Nature Chemistry*, presents groundbreaking insights into the ultrafast dynamics of electron transfer in molecular systems using attosecond extreme-ultraviolet pulses. This study not only cultivates a greater understanding of the electron-nuclear interplay in donor-acceptor molecules but also emphasizes the importance of precise temporal measurement in chemical processes.
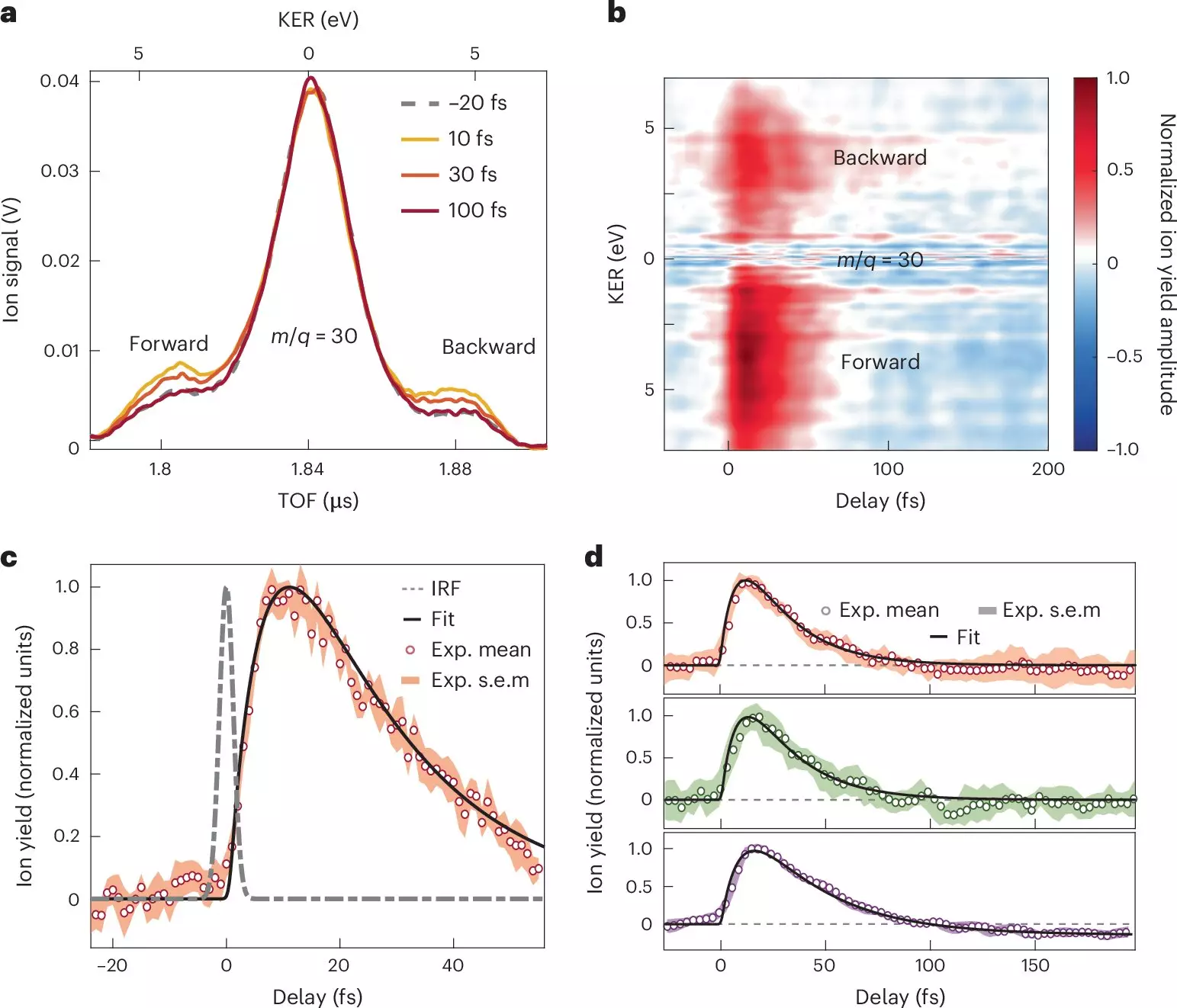
By focusing their study on nitroaniline molecules subjected to attosecond pulses, the research team has successfully captured and analyzed the earliest stages of charge transfer with remarkable accuracy. This work deploys a sophisticated array of techniques, including attosecond extreme-ultraviolet-pump alongside few-femtoseconds infrared-probe spectroscopy, combined with advanced many-body quantum chemistry calculations, to elucidate the rapid dynamics at play.
The research unveiled vital information regarding the timeframes involved in electron transfer from the amino group’s electron donor. Observations indicated that this transfer occurs in less than 10 femtoseconds, driven by a synchronous movement of both nuclei and electrons. Following this initial transfer, a relaxation phase emerges, spanning a sub-30-femtosecond interval, during which the nuclear wave packet disperses. These findings provide critical insights into how nuclear motion influences electron transfer processes and, consequently, the interaction between donor and acceptor components within molecules.
Importantly, the study outlines the timing required for charge migration from the donor unit to the adjacent bond associated with a benzene ring, as well as the structural alterations that accompany these processes. The researchers argue that their innovative findings could refine conventional understandings of charge migration in organic molecules, subsequently enriching the theoretical frameworks that inform these phenomena.
The implications of this study extend far beyond the laboratory, hinting at transformative potential in both theoretical research and practical applications within the realm of attosecond science. By illuminating the complexities of molecular dynamics, the authors set the stage for future research aimed at deepening our understanding of chemical processes at the quantum level. Such advancements could pave the way for innovations in energy capture, molecular electronics, and beyond. Through further exploration of these intricate dynamics, scientists may soon unlock new methods for harnessing and manipulating light-matter interactions, ultimately enriching the scientific community’s grasp of our universe at its most fundamental levels.

Leave a Reply