The evolution of energy storage technology hinges on the continuous improvement of battery systems, particularly solid-state batteries that promise higher performance and safety. A pivotal element in this quest lies in the use of lithium and sodium metal anodes, which have garnered significant attention due to their high capacity and potential advantages. Understanding the microstructure of these reactive metals could revolutionize solid-state batteries, enhancing their electrochemical properties and overall functionality.
Despite their potential, the practical application of lithium and sodium in batteries has remained fraught with challenges, primarily due to their chemical reactivity. The surfaces of these metals are prone to rapid oxidation, which leads to the formation of thick reaction layers that obscure their microstructure. Until recently, this has posed significant hurdles for researchers aiming to gain insights into how these materials function at a microscopic level. The reactivity of these alkali metals not only complicates the microstructural analysis but also raises concerns regarding the stability and safety of the battery systems that incorporate them.
In an unprecedented advancement, researchers from Justus Liebig University Giessen (JLU) collaborated with teams from the University of California, Santa Barbara, and the University of Waterloo to develop a novel methodology that allows for the detailed analysis of lithium and sodium metal structures. By employing a series of well-designed preparation and analysis techniques under inert gas environments and low temperatures, they achieved a breakthrough in the characterization of these metals.
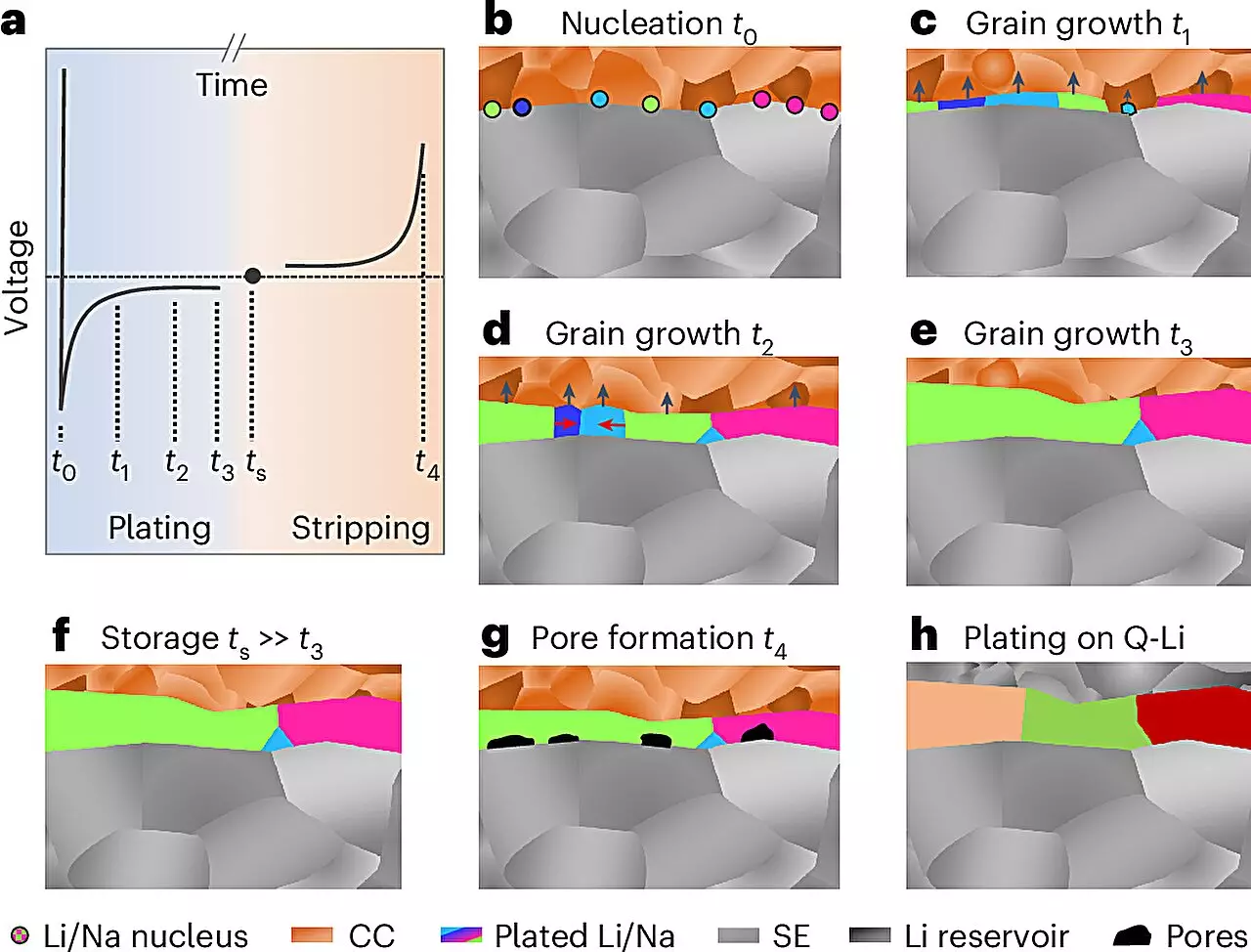
At the heart of their approach was the use of electron backscatter diffraction (EBSD), a sophisticated imaging technique that enabled the researchers to visualize the internal structures of metal layers deposited through electrochemical methods. This method sheds light on the dimensions of the grains within metal layers—crucial for understanding the growth mechanisms that govern these metals’ behavior during charge and discharge cycles.
Implications of Microstructure Insights
The findings from this research provide vital new knowledge about the microstructures of both lithium and sodium metals, revealing grain sizes and other structural features that impact how these metals perform in battery systems. This enhanced understanding could lead to more controlled growth processes, potentially minimizing the formation of undesirable structures such as dendrites—spiky formations that can lead to short circuits during battery operation.
Prof. Jürgen Janek, a leading figure in this research, indicated that the results could significantly enhance efforts within the POLiS (Post-Lithium Energy Storage) excellence cluster, particularly in exploring sodium-based batteries as viable alternatives to lithium systems. The ability to control and predict the behavior of these metals is not only crucial for advancing their performance but also for addressing the ongoing challenges posed by traditional lithium-ion technologies.
Future Prospects for Solid-State Batteries
The implications of these research advances extend beyond just lithium and sodium anodes. The exploration of ceramic solid electrolytes offers a pathway to incorporate these metals into high-performance battery architectures safely. However, the challenge remains in mitigating the physical deformation of the metal electrodes during cycling, which can adversely affect both charging and discharging efficiency.
The potential for solid-state batteries to serve as efficient, powerful, and safe energy storage solutions seems closer to fruition, thanks in part to insights gathered about the microstructural nuances of lithium and sodium. Future developments could integrate strategies that only allow for metal formation in controlled settings, thus sidestepping many of the hazards associated with using reactive alkali metal foils.
The collaborative efforts from the international research team underscore the importance of interdisciplinary approaches in tackling complex scientific challenges. By coming together, specialists in materials science and electrochemistry have not only expanded the scientific knowledge surrounding alkali metals but also laid the groundwork for advancements that could reshape the energy landscape. As solid-state battery technology continues to evolve, findings such as these will be instrumental in creating the next generation of energy storage solutions capable of meeting the growing demands of modern technology. Through ongoing research and innovation, the dream of building safer, more efficient, and longer-lasting batteries may soon become a reality.


Leave a Reply