In the realm of biological chemistry, research has predominantly targeted the prominent components that sustain life; from the mechanisms of protein folding to the intricacies of genetic expression and electrical signaling pathways. These areas have yielded fruitful insights into how irregularities can lead to various diseases. Nevertheless, a new frontier is emerging, bringing to light the significance of a lesser-known cellular structure known as biological condensates. These intriguing structures, akin to droplets of oil in water, form compartments within cells without conventional membrane barriers. Recent findings reveal that biological condensates play a pivotal role in influencing cellular behavior in ways previously overlooked.
Biological condensates are known for their ability to segregate or unite proteins and molecules, thereby impacting their functional activity. The unique property of these cellular constructs is their capacity to generate alternate energy sources, which may enhance specific biochemical processes. Historically, investigations into condensates have largely concentrated on their immediate environments, sidelining the broader implications of their existence. Two noteworthy institutions, Duke University and Washington University in St. Louis, have joined forces in a groundbreaking study published in the journal Cell on September 10. Their research indicates that biological condensates can influence cellular activities far beyond their localized spheres, acting as modulators of the cell’s internal electric activity.
The research led by Duke’s Lingchong You elucidates a fascinating mechanism of cellular interaction: biological condensates appear to possess a “wireless connection” with their surroundings. This connection enables condensates to influence cellular processes at a systemic level. By demonstrating the electrochemical dynamics governing this interaction, the researchers uncovered a potential pathway through which cells can alter their membrane properties—a crucial trait that ultimately affects how cells respond to external stimuli, including antibiotic exposure. Lingchong You’s assertion highlights the importance of these findings: “Beyond demonstrating the electrical mechanisms, we’ve shown that condensate formation can enhance cell tolerance to specific antibiotics while making them more susceptible to others.”
Biological condensates assume a sponge-like role within the cellular milieu, capturing a diverse range of biomolecules, from proteins and enzymes to ions. This selective entrapment can lead to ion concentration imbalances, generating a positive or negative charge. Such electrostatic variances are significant as they can alter the electric potential of the cellular membrane and the overall electrochemical landscape within the cell. Consequently, these changes affect various biological processes vital to the cell’s interaction with its environment, unraveling a new understanding of how condensates contribute to cellular physiology.
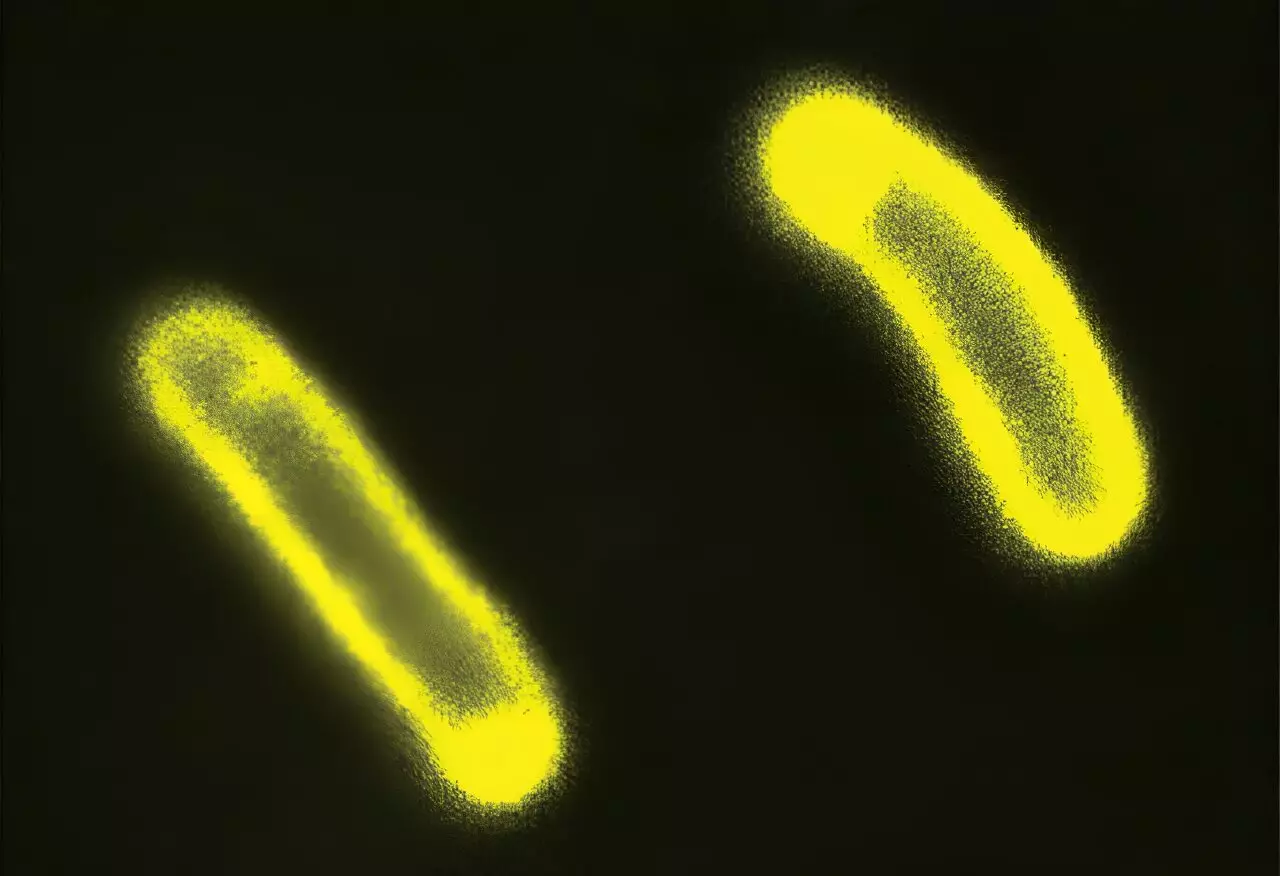
The study undertook a rigorous examination of the relationship between condensate formation and bacterial survival in the face of antibiotics. By inducing stress in E. coli colonies, researchers prompted the creation of internal condensates, subsequently observing changes in the electrical charges of cellular membranes. Results indicated that certain membranes developed a heightened negative charge, which played a direct role in bacterial response to the charged antibiotics. This pivotal observation posits that biological condensates are not merely passive structures—they actively influence the cell’s resistance strategies against antimicrobial agents.
The implications of this research extend far beyond what has been uncovered. Ashutosh Chilkoti’s comment about the potential breadth of condensate effects underscores the necessity for further investigations. The electrical characteristics that these structures impart could illuminate various cellular behaviors, suggesting that there is still much to learn about their roles in gene regulation and other fundamental biological processes. The research team firmly believes that their findings are merely a starting point—an intriguing opening that beckons additional exploration into the far-reaching impacts of biological condensates.
As the scientific community delves deeper into the nuances of biological condensates, it is becoming increasingly clear that these cellular structures hold a treasure trove of insights about cellular dynamics and interactions. The emerging perspective that condensates can modulate processes beyond their immediate vicinity opens a new chapter in biochemical exploration. By redefining our understanding of cellular machinery and the electrodynamics at play, researchers are set to uncover novel mechanisms that could fundamentally change our approach to understanding health and disease, particularly in the context of antibiotic resistance. The journey ahead promises to be as enlightening as it is essential.

Leave a Reply