The intricate web of chemical processes occurring in Earth’s atmosphere continues to intrigue scientists. Recent groundbreaking research from the Leibniz Institute for Tropospheric Research (TROPOS) in Leipzig has illuminated a previously elusive compound: sulfurous acid (H2SO3). This remarkable finding, reported in the esteemed journal Angewandte Chemie, challenges conventional understanding and opens new avenues for atmospheric science.
Historically, sulfurous acid has been shrouded in mystery. While sulfuric acid (H2SO4) is well-studied and understood, H2SO3 has generally been deemed difficult to generate in isolation. Textbooks posit that it might form in aqueous solutions of sulfur dioxide (SO2); however, previous attempts to isolate H2SO3 in this state have fallen short. The only earlier detection of this compound was achieved back in 1988, when Helmut Schwarz’s team at TU Berlin managed to observe it under vacuum conditions, although with an exceedingly brief lifespan estimated at just 10 microseconds.
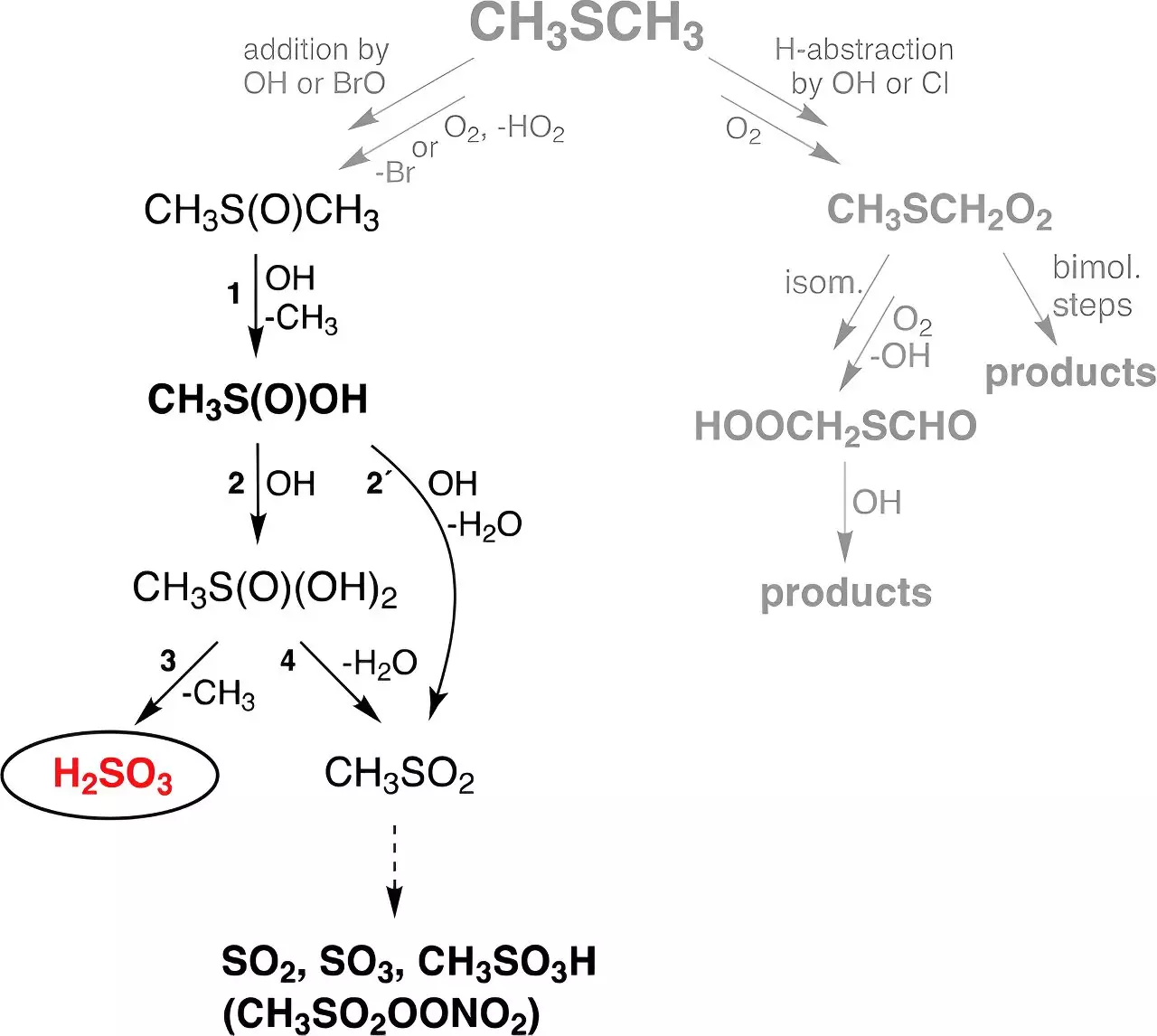
Despite its perceived instability, theoretical frameworks had suggested that H2SO3 could be a product of gas-phase reactions involving hydroxyl (OH) radicals and dimethyl sulfide (DMS). DMS, a major source of biogenic sulfur emanating from marine ecosystems, produces vast quantities of sulfur each year—approximately 30 million tons. The potential for novel pathways to H2SO3 formation in the atmosphere could have significant implications.
The recent study conducted at TROPOS utilized sophisticated flow reactors that replicate atmospheric conditions to explore the formation of H2SO3. Remarkably, researchers demonstrated a stable existence of sulfurous acid in gas phase for up to half a minute, irrespective of humidity levels. This discovery is significant, as it suggests that H2SO3 might exist longer in the atmosphere than previously conjectured, thereby influencing various chemical processes.
Dr. Torsten Berndt, who spearheaded the research, expressed awe at the clear signals from H2SO3 detected via their advanced spectroscopic methods, especially given that scientists had previously considered it practically non-existent in isolation. This unexpected yield exceeded theoretical predictions, hinting at the complex dynamics of atmospheric chemistry.
In light of the experimental findings, researchers incorporated this new reaction pathway into a global chemistry-climate model. The model simulations predict the annual global formation of around 8 million tons of H2SO3, a figure that significantly surpasses the anticipated amounts derived from traditional sulfuric acid generation pathways. This new understanding implies that the atmospheric chemistry of sulfur is far more intricate than previously acknowledged.
Dr. Andreas Tilgner and Dr. Erik Hoffmann noted that the reaction pathway identified produces approximately 200 times more mass of H2SO3 compared to sulfuric acid from dimethyl sulfide. This insight not only enhances our grasp of the sulfur cycle within the atmosphere but also prompts further inquiries about H2SO3’s behavior once formed.
Nonetheless, numerous questions remain. While this research suggests a degree of stability for sulfurous acid in the gas phase, the implications of its interactions with trace gases are still largely unknown. The specifics regarding its reactivity with water vapor also lack comprehensive understanding. Dr. Berndt emphasized the necessity for more refined experimental setups and methodologies to further investigate these dynamics, suggesting that the role of H2SO3 in atmospheric processes requires rigorous exploration.
The detection of sulfurous acid underscores an exciting era of discovering new reaction pathways and experimentally validated compounds that were previously elusive. With the advancement of detection technology—including mass spectrometers capable of identifying products contingent upon the presence of a staggering number of molecules—scientists can delve deeper into the complexities of atmospheric chemistry.
As researchers continue to unveil these critical aspects of atmospheric chemistry, the implications go beyond academic curiosity. Understanding how sulfurous acid and related compounds behave within our atmosphere is vital for evaluating environmental changes, pollution, and global climate dynamics. The quest for knowledge in atmospheric chemistry is not merely an intellectual endeavor; it is a necessary pursuit for fostering a more comprehensive understanding of Earth’s intricate systems and their responses to anthropogenic influences.
The recent discovery of sulfurous acid in the atmosphere serves as a potent reminder of the ever-evolving narrative of chemical science. The interplay between innovative experimental designs and sophisticated analytical techniques will undoubtedly pave the way for significant advancements in our comprehension of the atmospheric phenomena that shape our planet. This journey of inquiry promises to reveal not only the hidden intricacies of sulfurous compounds but also the critical role they play in the Earth’s climatic symphony.

Leave a Reply