Ovarian cancer, particularly its most aggressive form known as high-grade serous ovarian carcinoma (HGSOC), remains a leading cause of cancer-related deaths among women. This form of cancer has alarmingly high mortality rates, with many patients succumbing within five years of diagnosis, primarily due to late-stage detection. The challenge lies not just in identifying this insidious disease but also in uncovering its origins. Recent discoveries from research conducted on mice point to a substantial advancement in understanding where HGSOC begins, potentially offering new avenues for early diagnostics and treatment.
For years, medical professionals operated under the assumption that ovarian cancers predominantly originated within the ovaries. However, mounting evidence over the past decade has shifted this paradigm, suggesting that a significant number of these cancers may actually arise from the fallopian tubes—specifically, the oviducts. Research has pinpointed genetic lesions at the distal ends of these tubes that are closely linked to tumor development in the ovaries. Despite these findings, the precise cellular origin of HGSOC has remained elusive, creating a significant barrier to improving patient outcomes.
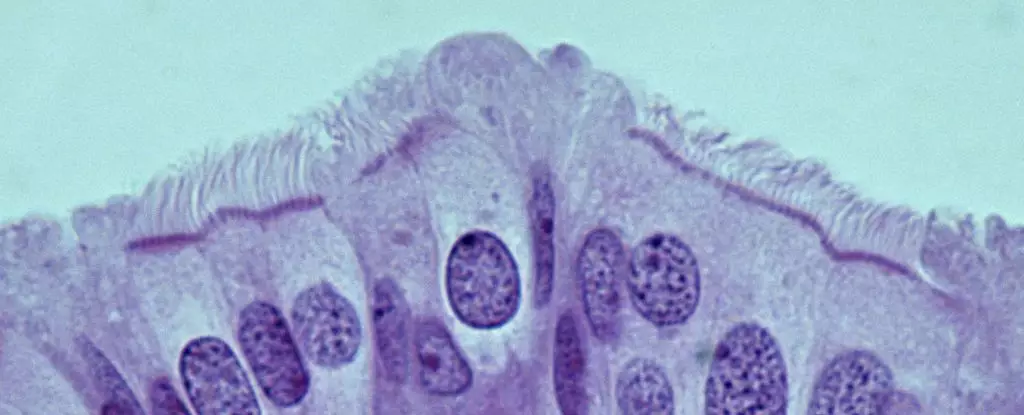
Led by Cornell University pathologist Alexander Nikitin, a recent study has made significant strides in localizing the cells that might lead to HGSOC within mouse models. The researchers meticulously cataloged the diverse cell types residing in the oviduct, revealing that the most cancer-prone cells were not the anticipated stem cells but rather pre-ciliated cells. These transitional cells serve a crucial role in the reproductive process by using hair-like structures to aid in the transportation of oocytes within the oviduct. This finding shifts the focus of future research towards understanding the unique properties of pre-ciliated cells and their susceptibility to cancer.
An intriguing aspect of this research is the discovery that two specific genetic mutations commonly linked to HGSOC disrupt normal function in these pre-ciliated cells. The presence of these mutations leads to the formation of cancers by impairing cilia, structures vital for the cells’ function. The link between cilia formation dysregulation in the uterine tubes and ovarian cancer not only sheds light on HGSOC’s initiation but also broadens our understanding of cancer biology, as similar ciliary dysfunctions have been implicated in other malignancies, including pancreatic cancer. Identifying a similar mechanism across different cancers could revolutionize approaches to prevention and treatment.
The translational potential of these findings is profound. If the identified cancer-prone cells in mice correspond to similar cells in human fallopian tubes, it opens up possibilities for early detection strategies. Currently, about 80% of HGSOC cases are diagnosed at advanced stages, severely limiting treatment options. However, understanding the cellular origins and mechanisms of HGSOC could lead to the development of diagnostic markers that facilitate earlier intervention.
While these findings are promising, they are merely the stepping stones towards a more comprehensive understanding of ovarian cancer. Future studies must delve deeper into the mechanisms of tumor formation and explore the roles of other genetic mutations associated with HGSOC. Moreover, investigating how these mechanisms differ or align with those in human tissues will be critical in bridging the gap between animal models and clinical applications.
The journey toward eradicating ovarian cancer is fraught with challenges, yet breakthroughs in the understanding of its origins provide a beacon of hope. The research laying a foundation in mice presents an important opportunity for early detection and treatment strategies that could ultimately save lives. As the scientific community continues to unravel the complexities of cancer biology, each discovery serves as a pivotal component in the quest to outsmart this formidable disease.

Leave a Reply