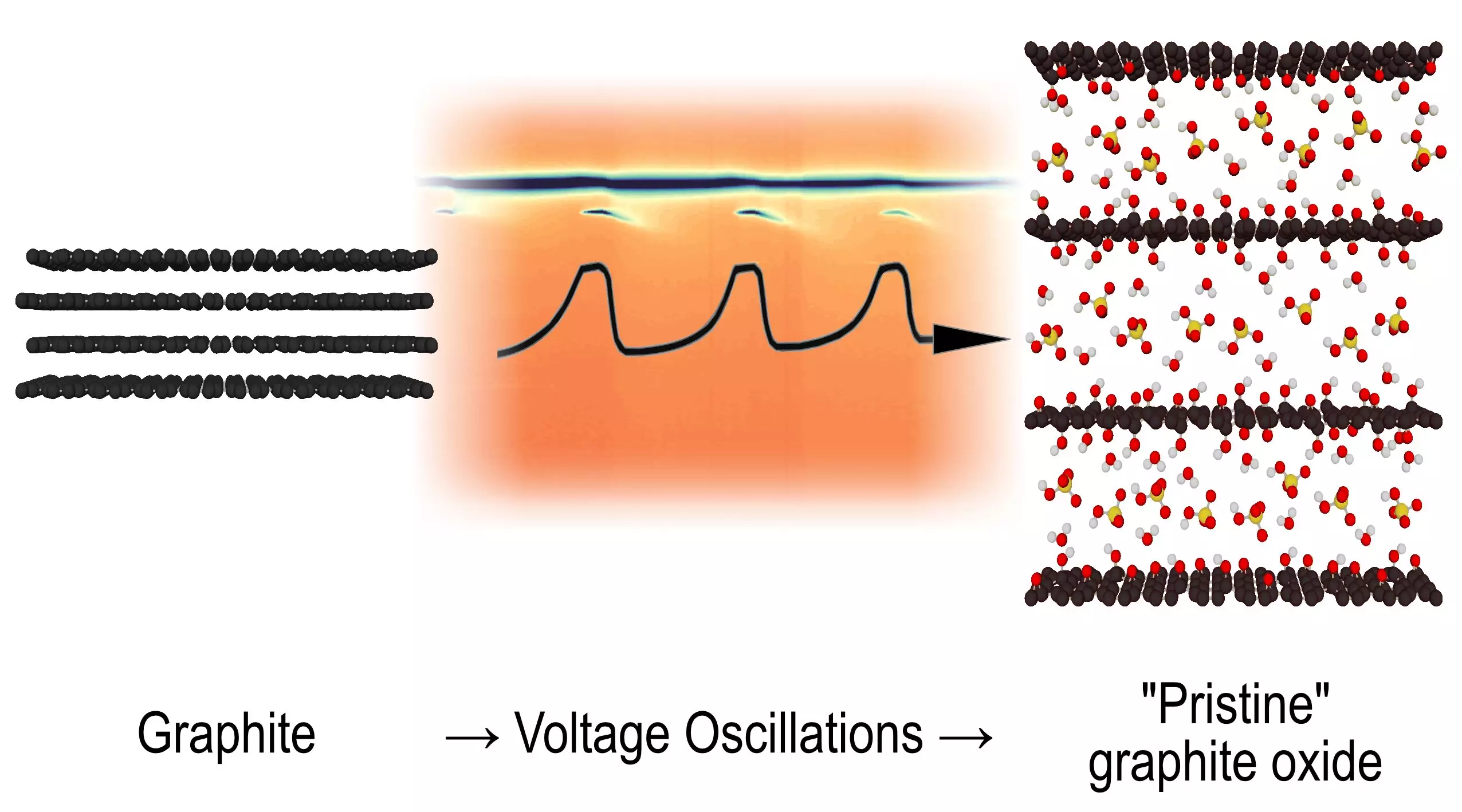
For five decades, chemists have wrestled with the perplexing behaviors of graphite as it undergoes electrochemical oxidation to form graphite oxide. Recent breakthroughs from Umeå University have finally shed light on this intricate process. The researchers’ keen observations have revealed unexpected intermediate structures that fluctuate during galvanic reactions, suggesting a previously undocumented form of oscillating reaction. Published in the prestigious journal Angewandte Chemie, this study not only provides clarity on graphite oxidation but also expands the broader understanding of oscillating chemical reactions.
Oscillating chemical reactions challenge the conventional understanding of chemical stability and dynamics. Traditionally, chemists have analyzed reactions primarily from a linear perspective—inputs lead to outputs in a predictable manner. However, oscillating reactions defy this simplicity, exhibiting cyclical color changes or concentration variations in solutions, thereby creating a rich tapestry of chemical behavior. These dynamical systems have been integral for scientific explorations, yet their intriguing nature has encouraged skepticism, particularly concerning their applicability in inorganic chemistry.
Alexandr Talyzin, a prominent physicist, emphasized the longstanding enigma of voltage-induced oscillations at graphite electrodes submerged in sulfuric acid. Although the formation of graphite oxide—characterized by layered arrangements of graphene oxide—was established, the precise structural transformations occurring during the oscillation cycles had eluded explanation for years. The breakthroughs achieved by Talyzin and his colleagues have thus positioned their work at a critical juncture in the evolution of chemical research.
The dawn of new synchrotron technologies has unlocked capabilities previously unimagined in the field of chemistry. Utilizing rapid X-ray diffraction scans, researchers can now capture real-time, split-second snapshots of material structural changes during oxidation reactions. The ability to visualize these fleeting intermediate structures has illuminated the complex choreography of chemical processes. What the team discovered was revolutionary: a distinct intermediate phase that not only appeared and vanished in a rhythmic manner but did so consistently across multiple cycles.
This unexpected revelation ushered in a paradigm shift for researchers. Bartosz Gurzeda, the leading author of the study, even produced a captivating video that showcases these ongoing structural transformations, providing a palpable visual reference to their findings. Such advancements in technology not only facilitate deeper explorations into the minutiae of chemical reactions but also inspire new methodologies for understanding the dynamics of other oscillatory systems.
The implications of this research extend far beyond the confines of inorganic chemistry. Oscillation phenomena are omnipresent in living organisms; countless biochemical processes rely on complex oscillatory feedback mechanisms that maintain homeostasis and support life. This discovery challenges the notion that oscillation reactions are limited to biological contexts, pushing the boundaries of our understanding of chemical kinetics and unlocking potential avenues for further study.
Talyzin posits that the insights gleaned from this study could inspire fresh theoretical frameworks and models in the realm of chemistry. The legacy left behind by Ilya Prigogine, who won a Nobel Prize for his foundational theories of oscillating reactions, illustrates the profundity with which a single discovery can reverberate through the scientific community. Following this tradition, Talyzin and his team hope that their observations will foster new theories that explain this novel oscillating reaction type and unveil additional examples of similar phenomena.
In an age where scientific advancements often stem from daring explorations into the unknown, this research positioned at the intersection of theoretical chemistry and practical experimentation beckons further inquiry. With our evolving comprehension of oscillating reactions and their implications, the scientific community stands on the precipice of new discoveries that could recast our understanding of chemical systems entirely. As we embark on this journey, the excitement of uncovering new realms within chemistry continues to inspire researchers to probe the mysteries that remain. The canvas of chemical phenomena is vast, and with every new discovery, we inch closer toward unlocking its secrets.

Leave a Reply