Recent research conducted by an interdisciplinary team from prominent institutions such as the Fritz Haber Institute, Sorbonne University, and Uppsala University presents a groundbreaking advance in our comprehension of how ions behave in solutions. Their paper, “The solvation shell probed by resonant intermolecular Coulombic decay,” published in the esteemed journal *Nature Communications*, shines a light on the intricate dynamics of solvation shells – the thin layers of solvent molecules that envelop dissolved ions or particles.
For years, solvation shells have eluded precise study, largely due to their complex nature. When a solute dissolves, the surrounding solvent molecules do not behave uniformly. The molecules closest to the ions often exhibit markedly different properties compared to their bulk counterparts. This disparity creates a challenge for scientists seeking to understand the nuances of solvent-solvent interactions at the molecular level. The research team’s innovative approach aims to demystify this phenomenon, providing unprecedented insights into the solvation process.
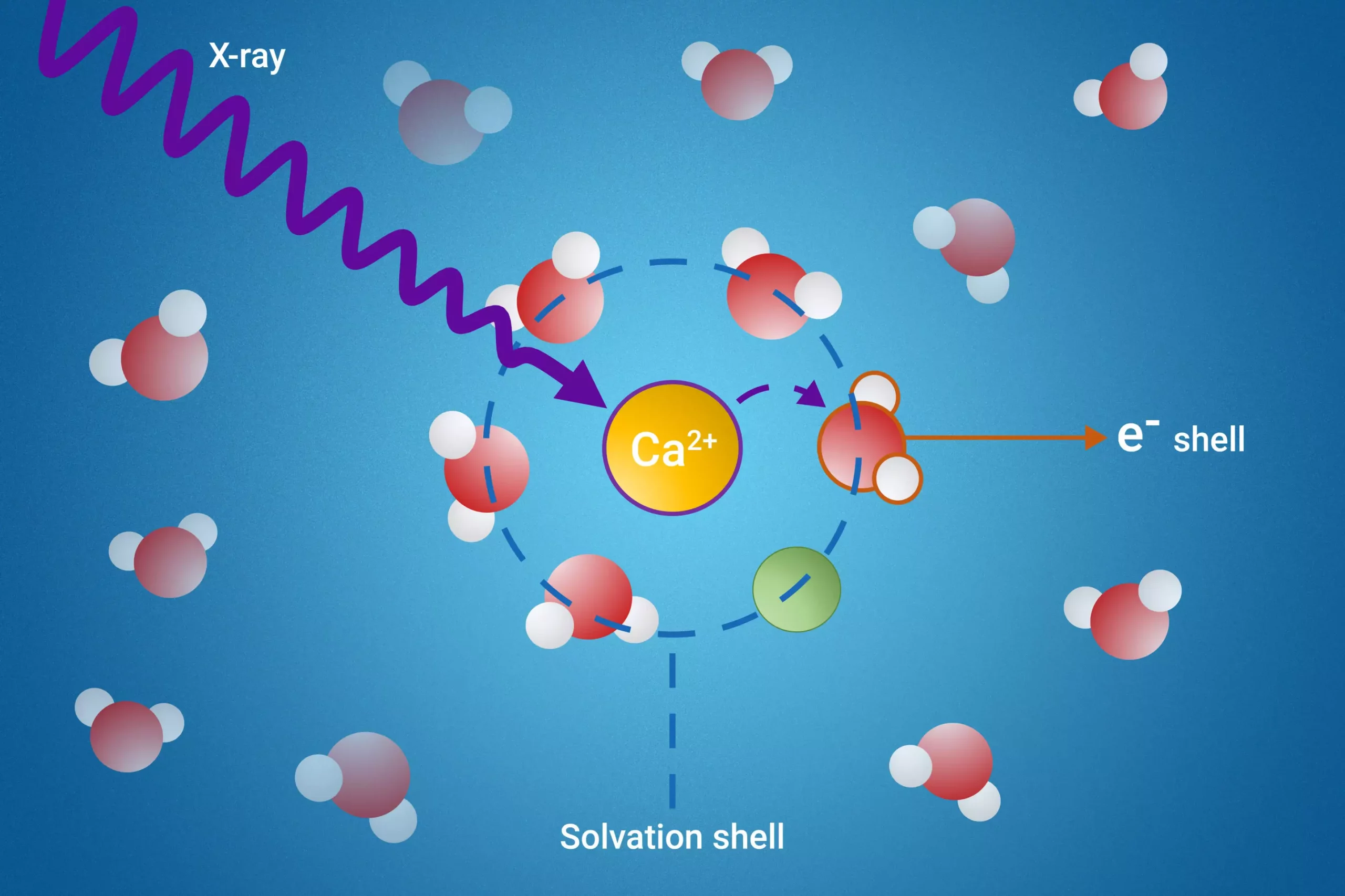
The breakthrough method developed by the researchers is called resonant intermolecular Coulombic decay (ICD). This technique utilizes high-energy X-rays to excite the molecules, allowing scientists to scrutinize how these solvent molecules interact with each other during the decay phase. The ability to observe these interactions directly addresses one of the primary challenges in studying solvation shells: the difficulty in isolating and studying the specific solvent molecules directly involved in the solvation process.
For the first time, scientists were able to accurately measure electron binding energies for water molecules located within the first solvation shell of the dissolved particles. This achievement is paramount because it opens up new avenues of research previously considered unattainable. It not only enhances our understanding of molecular interactions but also enables more accurate predictions regarding ion behavior in various environments.
The implications of understanding solvation shells extend across multiple scientific disciplines. In fields such as chemistry and biology, insights into solvation can unravel fundamental biological processes and chemical reactions. For instance, the way ions interact within biological systems is pivotal for enzyme activity and cellular functions. Similarly, in materials science and electrochemistry, knowledge of solvation shell dynamics can inform the design of more efficient batteries and catalysts.
Furthermore, from an atmospheric science perspective, understanding the solvation processes can contribute to more accurate models for predicting environmental behavior and reactions that occur in our atmosphere. As the research unfolds, the potential applications of these findings will likely inspire new studies and innovations that transcend traditional disciplinary boundaries.
As this research garners attention, it becomes evident that the method presented not only enhances our understanding of solvation shells but also paves the way for future explorations. The interdisciplinary nature of this study exemplifies how collaborative efforts can lead to significant advancements in science. Anticipating the further applications of this technique, researchers are poised to delve deeper into the mysteries of solvation phenomena, potentially revolutionizing our approach to studying molecular interactions across a variety of fields.

Leave a Reply