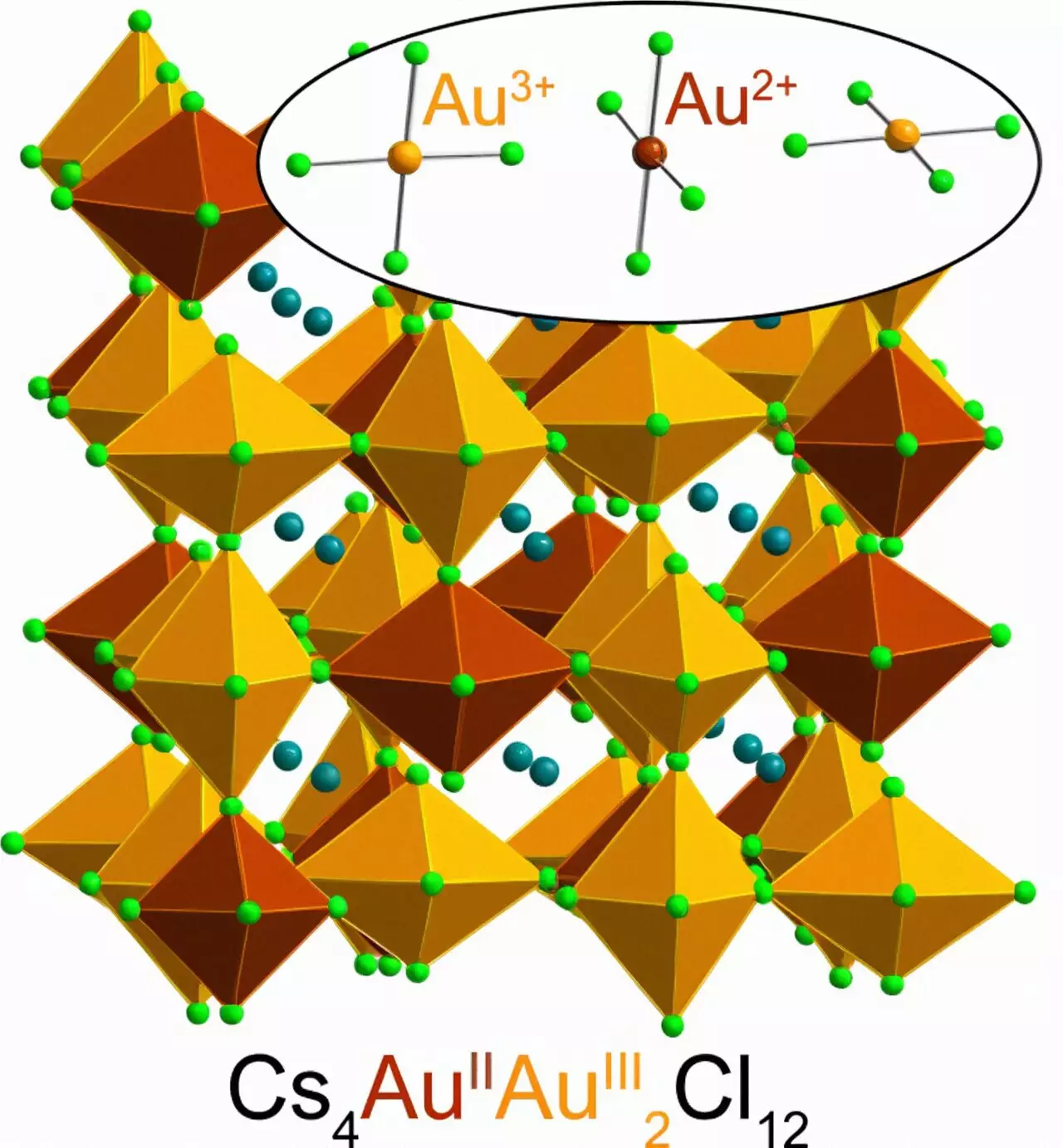
In a groundbreaking study, researchers from Stanford University have achieved a scientific feat previously thought to be impossible. For the first time ever, they have successfully created and stabilized a highly elusive form of gold known as Au2+ using a halide perovskite. What makes this discovery all the more remarkable is the simplicity and speed with which this rare form of gold can be synthesized, using readily available ingredients at room temperature. This opens up a world of possibilities for the development of more efficient solar cells, light sources, and electronic components.
Lead author Kurt Lindquist, a postdoctoral scholar in inorganic chemistry at Princeton University, revealed that the synthesis of Au2+ perovskite was stumbled upon during an experiment focusing on magnetic semiconductors for electronic devices. By combining cesium chloride, Au3+-chloride, water, and a dash of hydrochloric acid infused with vitamin C, Lindquist successfully created the stable Au2+ perovskite. Astonishingly, the entire process could be completed in just five minutes at room temperature, resulting in a dark green, almost black powder with a significantly increased weight due to its gold content.
Gold has been treasured for centuries due to its scarcity, unique color, and chemical inertness. However, the formation of Au2+ is extremely rare. The root cause lies in the relativistic effects proposed by Albert Einstein’s theory of relativity. When particles, such as gold atoms, approach the speed of light, they become heavier. This phenomenon affects heavy elements like gold, which possess large atomic nuclei composed of numerous protons. The positively charged atomic nuclei surround themselves with heavy, tightly bound electrons, causing gold to absorb blue light and exhibit its characteristic yellow color. As a result of these changes in electron arrangement and energy levels, gold primarily occurs as Au1+ or Au3+, with the formation of Au2+ being highly unlikely.
Unlocking the Mystery: Stability of Au2+ Perovskite
Through meticulous testing, including spectroscopy and X-ray diffraction, Stanford researchers confirmed the presence of Au2+ in the perovskite and shed light on its crystal structure and light absorption properties. Notably, the discovery of Au2+ perovskite adds another chapter to the century-old story of chemistry and physics, connecting with the pioneering work of Linus Pauling. Coincidentally, Pauling investigated gold perovskites containing Au1+ and Au3+ earlier in his career, advancing our understanding of these materials. The fact that vitamin C, one of the key ingredients required for the stable synthesis of Au2+, was also studied by Pauling adds a fascinating connection to the research.
The successful creation of Au2+ perovskite opens up new avenues for research and development in the fields of magnetism and conductivity. The unique electronic structure of Au2+ could be harnessed in various applications, where electrons can easily transition between Au2+ and Au3+ within the perovskite structure. The team of researchers led by associate professor Hemamala Karunadasa plans to further investigate and fine-tune the chemistry of Au2+ perovskite, aiming to unleash its full potential in magnetism and conductivity-based technologies.
The discovery and stabilization of Au2+ perovskite by Stanford researchers have marked a significant milestone in materials science. This breakthrough has not only unraveled the mysteries surrounding rare gold formations but also presented a myriad of possibilities for the development of next-generation solar cells, light sources, and electronic components. With further exploration and refinement of the Au2+ perovskite’s properties, we may witness a revolution in magnetic and conductive materials used in various technological applications. The story of Au2+ perovskite adds an intriguing chapter to the rich history of chemistry and physics, connecting the work of modern-day scientists to the pioneering efforts of great minds like Linus Pauling.

Leave a Reply