Clathrate hydrates are unique structures that consist of water molecules forming a lattice around guest molecules. These complex formations play a crucial role in material science research due to their ability to alter physicochemical properties. One particular type of clathrate hydrate, known as Frank-Kasper (FK) phases, exhibits a geometric arrangement of close-packed tetrahedra, making their synthesis a challenging task.
A Breakthrough in Synthesizing Clathrate Hydrates
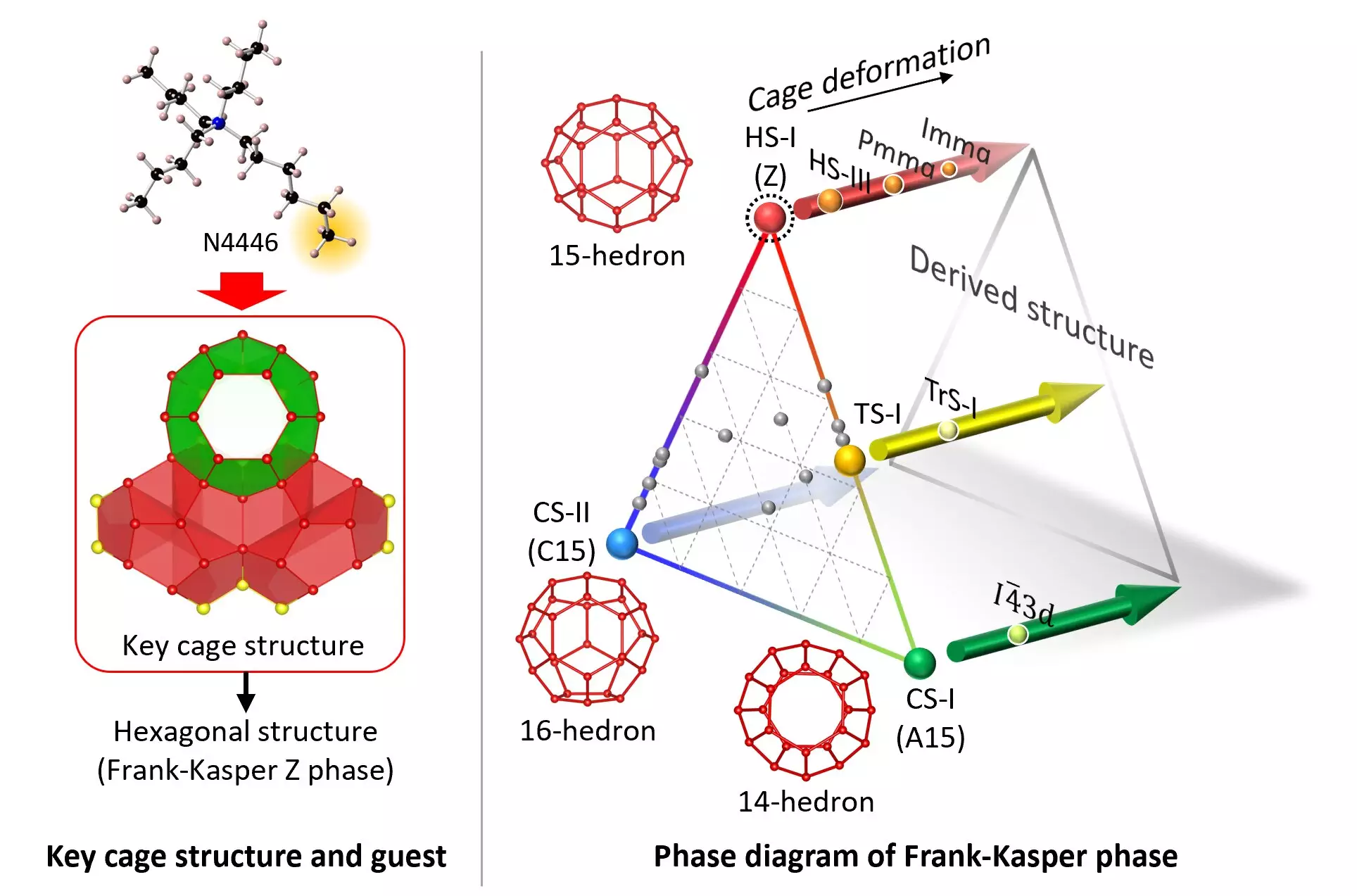
Recently, a team of researchers from Yokohama National University and the National Institute of Advanced Industrial and Science Technology (AIST) in Japan successfully synthesized a stable form of HS-I clathrate hydrate. This achievement marks a significant milestone in the field of material science, as the hexagonal structure of HS-I was previously deemed thermodynamically unstable and unattainable.
The research team discovered that fine-tuning the guest molecule, tri-n-butyl, n-hexylammonium chloride (N4446Cl), was essential in stabilizing the true form of the HS-I clathrate hydrate. Specifically, the n-hexyl chain within the guest molecule played a crucial role in creating the necessary water-molecule cage structure for the hexagonal crystal formation. By carefully selecting and adjusting the guest molecules, researchers were able to produce stable clathrate hydrates under various gas pressures and atmospheric conditions.
The successful synthesis of HS-I clathrate hydrate under ambient temperature and pressure conditions opens up new possibilities for material science exploration. This breakthrough paves the way for advancements in storage and transportation technologies for natural gas and synthetic fuels, carbon dioxide separation and recovery processes, and the development of novel materials with tailored properties. The ability to engineer clathrate hydrates with mixed FK phases has the potential to revolutionize various industries and accelerate innovation in material design.
The recent research conducted on clathrate hydrates has unlocked a world of possibilities for material science research and application. By understanding and manipulating the intricate structures of these compounds, researchers are poised to make significant advancements in energy storage, environmental conservation, and material development. The synthesis of stable HS-I clathrate hydrate serves as a stepping stone for future discoveries and innovations in the field, offering new avenues for sustainable technologies and cutting-edge materials.

Leave a Reply