Life displays a remarkable characteristic: the ability to self-organize. This peculiar property is fundamental to biological processes, particularly in the context of how living cells divide. At the Institute of Science and Technology Austria (ISTA), researchers have been uncovering the hidden mechanisms that allow bacterial cells to achieve this feat. Central to their research is a protein known as FtsZ, which plays a pivotal role in the formation of a division ring critical for bacterial cell reproduction. This exploration not only enhances our understanding of biological structures but also has profound implications for the development of synthetic materials.
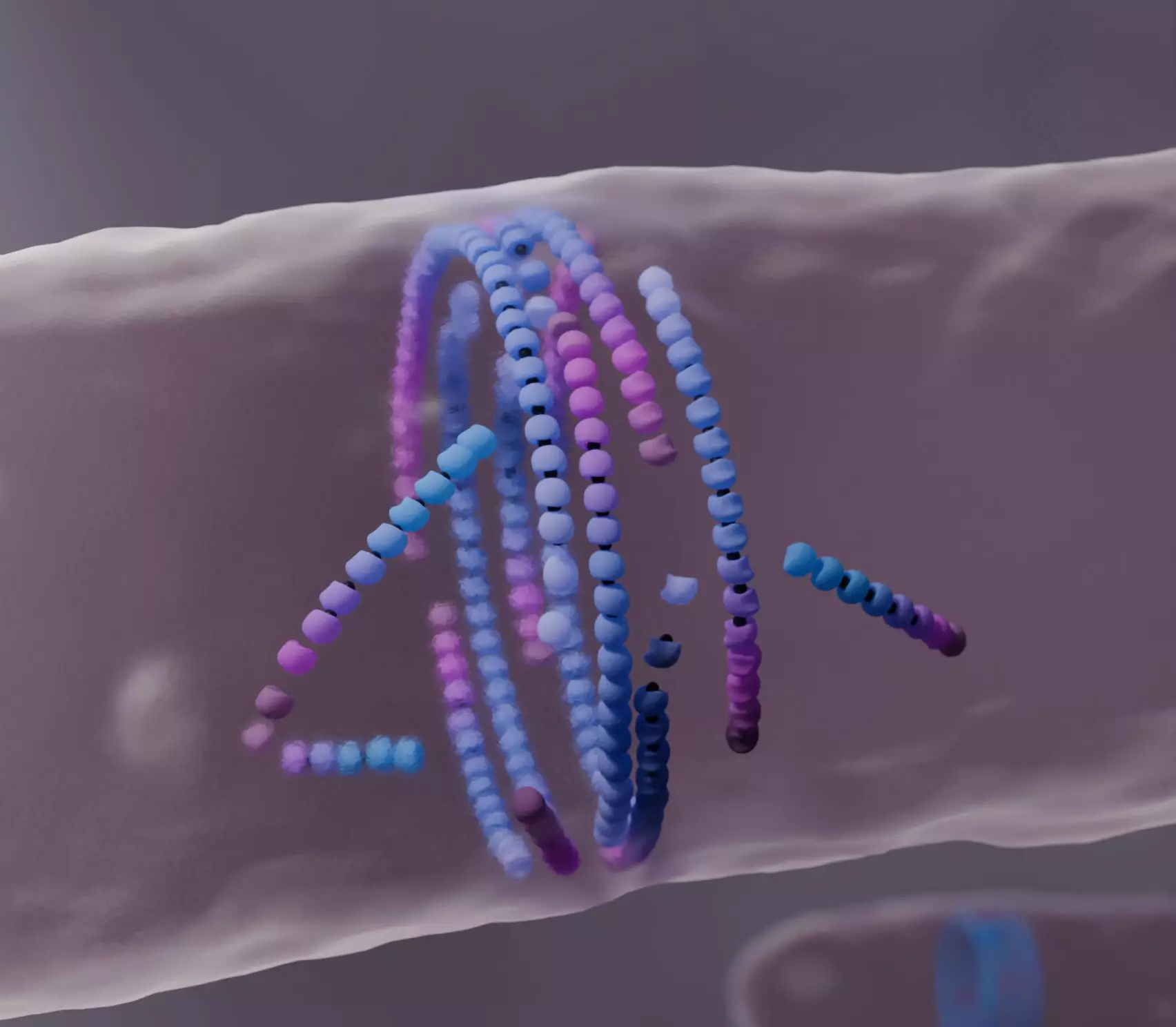
The FtsZ protein, akin to a molecular scaffold, self-assembles into a ring that helps segregate bacterial cells during division. Typically, the process involves the continuous addition of protein subunits on one end of the filament while simultaneously removing them from the other end, a phenomenon referred to as “treadmilling.” This self-organization is not just fundamental to bacterial life but is also observed across multiple life forms, including animals and plants. However, the mechanics of how FtsZ achieves such alignment during cell division remained elusive, prompting further investigation into the intricacies of its filament dynamics.
Recent findings led by Professor Anđela Šarić and her team reveal that misaligned FtsZ filaments exhibit a unique behavior when faced with obstacles. Instead of continuing to grow, these filaments undergo a process of “dying,” which ironically aids in the formation of well-aligned FtsZ rings. This mechanism of self-correction ensures the assembly of a structured and effective division ring at the cell’s center, crucial for proper cell reproduction. The concept of “dying to align” elegantly illustrates a sophisticated balance between life and the inherent tendencies of biological components to realign and effectively contribute to essential cellular functions.
The findings from Šarić’s group do not only advance our understanding of bacterial cell mechanics but also pave the way for innovative applications in material science. The research indicates that principles observed in biological active matter could inspire the creation of synthetic self-healing materials. In a world where materials either fail or degrade, mimicking the properties that allow FtsZ to reorganize could lead to significant advances in material longevity and functionality. It pushes scientists to consider how lifeless materials might adopt characteristics of living systems, specifically their ability to adapt and self-repair.
The collaboration between Šarić’s group and experimental teams at the University of Warwick and ISTA exemplifies the power of interdisciplinary research. The experimental validations, led by researchers such as Seamus Holden and Martin Loose, underscored the necessity of combining computational models with real-world observations. Their collective effort not only confirmed the computational predictions made by Šarić’s team, but it also illustrated the importance of dialogue among scientists from different fields in enhancing our understanding of complex biological phenomena.
Building upon these foundational insights, the Šarić group is poised to explore further dimensions of how the bacterial division ring contributes to the formation of the cell’s structure itself. This ongoing exploration could provide deeper insights into what constitutes “living matter” and how these principles can be harnessed for synthetic applications. As researchers delve deeper into the dynamic interplay of molecular interactions, the potential to create materials that mimic these biological processes becomes increasingly viable.
The study of FtsZ and its role in bacterial cell division illuminates a profound aspect of life’s complexity—self-organization in the face of adversity. By dissecting how misalignment leads to structural realignment through a process akin to “death,” researchers are not merely observing biological phenomena but are uncovering principles that could redefine material science and bioengineering. As science continues to decode the mysteries of life, the implications resonate far beyond the cellular level, offering new perspectives on sustainability, resilience, and the intriguing question of how the inanimate can mimic the living.


Leave a Reply