In a groundbreaking advancement, researchers have unveiled a method to synergistically combine the unique properties of DNA and proteins into biohybrid molecules. This innovative approach leverages the natural capabilities of bacteria, marking a significant leap in therapeutic development. Led by a dedicated team from the University of Illinois Urbana-Champaign, including biochemistry professor Satish Nair and postdoctoral researcher Zeng-Fei Pei, this discovery offers a fresh perspective on genetic and protein engineering. Their findings, published in *Nature Chemical Biology*, shine light on the potential for creating complex, targeted therapies with enhanced efficiency.
The fusion of nucleic acids and amino acids to form new biohybrid molecules holds immense promise for precision medicine. Nair describes the dual nature of nucleic acids, which like DNA and RNA, are essential for genetic makeup, and amino acids that form the structure of proteins. By integrating these elements, researchers aim to create precision drugs that can specifically target and interrupt disease pathways at the molecular level. Such advancements could revolutionize treatments by effectively shutting down transcription from mutated genes or inhibiting problematic noncoding RNA molecules that contribute to various diseases.
In achieving this fusion, the ability to construct biohybrid molecules represents more than mere scientific curiosity; it is a potential game-changer for tackling diseases that have been resistant to existing therapies. The precision in delivering these dual-action drugs creates a pathway to minimize off-target effects and enhance therapeutic efficacy, addressing a persistent challenge in drug development.
This transformative process was initially spurred by an unexpected finding. Nair and his team were originally investigating proteins that interact with metal ions when they stumbled upon research from the John Innes Centre in Norwich, England. Researchers Natalia Vior and Andrew Truman had observed a molecule that appeared to combine elements of DNA and protein, sparking a collaborative inquiry between the two teams. This partnership exemplifies the importance of interdisciplinary research and how a willingness to explore uncharted territories can lead to significant scientific progress.
Once the existence of this DNA-protein hybrid was confirmed, Nair and his collaborators dove deeper into understanding its molecular structure and formation mechanisms. This essential knowledge lays the groundwork for future applications, allowing for the scalable synthesis of biohybrid molecules, eliminating the hurdles of conventional methods that often rely on labor-intensive, chemical synthesis processes.
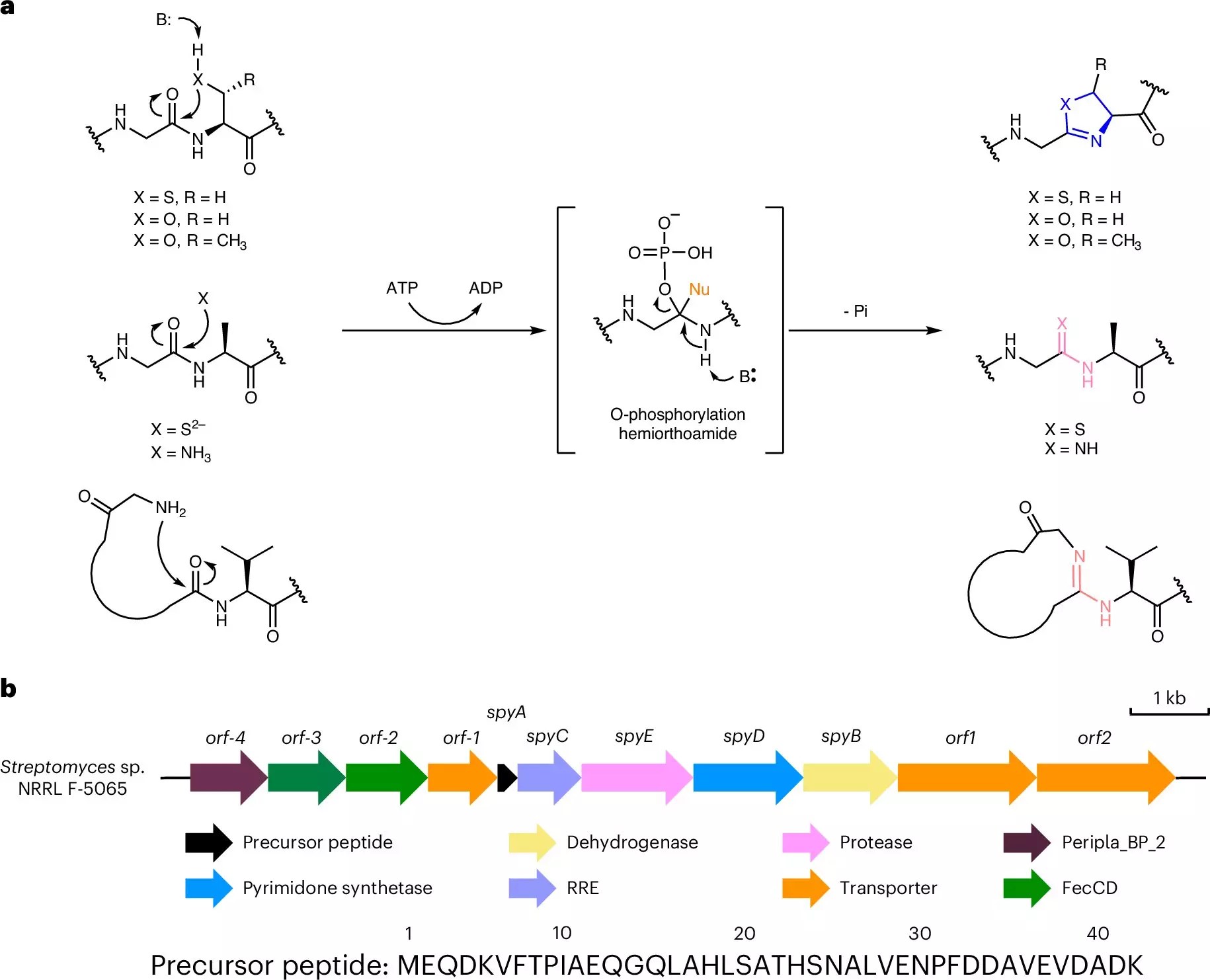
One of the most exciting aspects of this research is the novel use of bacterial enzymes to generate biohybrids efficiently. Nair’s team identified two specific bacterial enzymes that work synergistically to convert peptides into functional DNA-protein hybrids. The first enzyme, YcaO, initiates the modification of an amino acid within the peptide, resulting in a ring structure akin to the bases of nucleic acids. This crucial step paves the way for molecule pairing that is foundational in biological processes. The second enzyme operates as a protease, facilitating the truncation of the modified peptide into a functional hybrid.
The incredible simplicity of the system—requiring only three components: a peptide and two enzymes—speaks to a new standard in molecular biology. By demonstrating that E. coli can carry out this process, the research team has effectively opened the gateway for laboratories worldwide to adopt this methodology, streamlining the construction of vast libraries of biohybrid molecules that may be tested as prospective therapeutics.
With the foundational principles established and a method for rapid production identified, the scientific community is poised for a surge of investigation into biohybrid molecules. Nair’s enthusiasm for the project underscores an optimistic viewpoint: “Now, we’re off to the races.” This declaration speaks volumes about the potential for rapid advancements and the transformation of traditional drug discovery paradigms.
As research continues to unfold, the implications extend beyond mere academic inquiry. The practical applications of biohybrid therapy could transform clinical approaches to treating complex diseases, offering tailored solutions informed by the molecular mechanisms at play. Furthermore, the collaboration between biology and chemistry in this context underscores the emerging possibilities of hybrid scientific fields, transcending traditional boundaries. In summation, the work conducted by Nair and his colleagues embodies a paradigm shift in how we conceptualize and engineer therapeutic agents, heralding a bright future for molecular medicine.


Leave a Reply