In an era where environmental degradation and climate change are pressing concerns, the quest for sustainable solutions to pollution is more critical than ever. Hydrocarbons, which are prevalent in fuels and industrial emissions, constitute a significant component of harmful pollutants. They not only contribute to the toxic smog enveloping cities globally but also persist in ecosystems, posing grave risks to wildlife and human health. The breakthrough discovery by a team at UNIST, led by Professor Jaeheung Cho, represents a pivotal moment in the pursuit of cleaner technologies.
Advancements in Catalytic Design
The researchers at UNIST have ingeniously designed a catalyst that emulates the functionalities of natural metalloenzymes—complex biological catalysts that are renowned for their efficiency in oxidizing hydrocarbons. This novel catalyst offers a promising alternative to traditional methods that often require harsh conditions and contribute significantly to energy consumption. Published in the esteemed Journal of the American Chemical Society, this research marks a significant advancement in the field of catalytic chemistry, underscoring the potential of biomimetic approaches in environmental applications.
How the New Catalyst Works
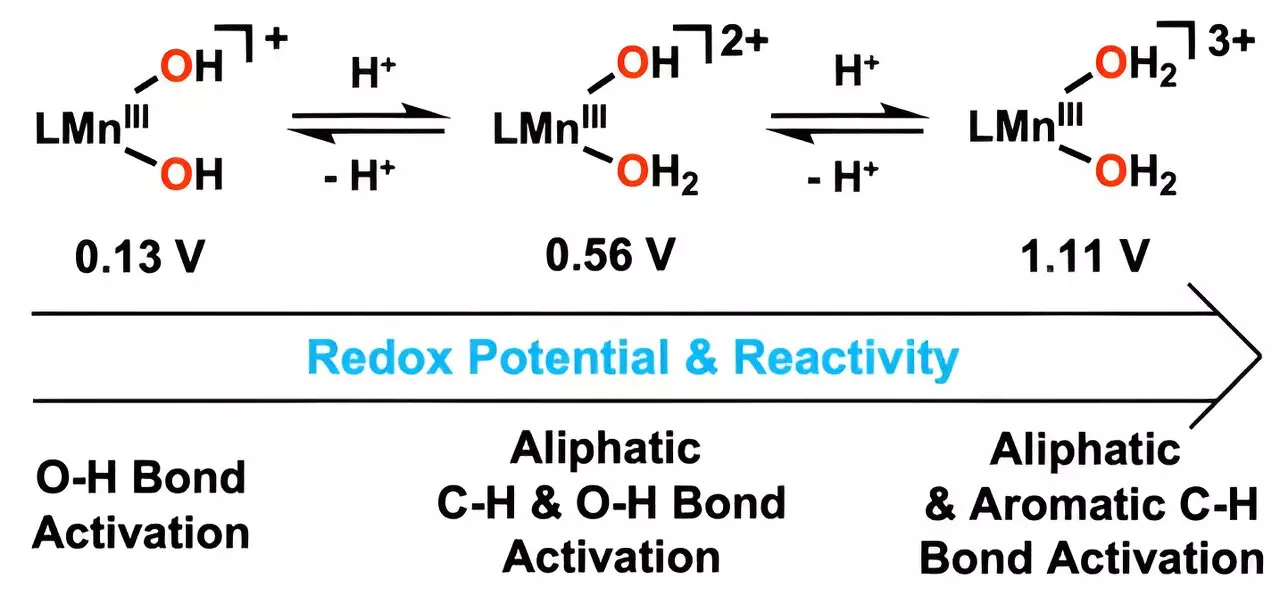
At the heart of this innovation lies the strategic inclusion of hydrogen ions, which react with hydroxo ligands to produce metal-bound water molecules. This clever modification is what enables the catalyst to target stubborn carbon-hydrogen (C-H) bonds at significantly lower temperatures compared to current catalytic methods. The efficiency gains are not merely incremental; they represent a transformative shift in how we can approach the oxidation of toxic substances in a sustainable manner. When the catalyst was tested with anthracene, a notoriously stable hydrocarbon, its ability to facilitate oxidation at mild conditions was not just notable—it showcased its potential to be a game-changer in environmental remediation.
Implications for Industrial Applications
Professor Cho’s work sheds light on a substantial breakthrough in industrial catalysis by manipulating manganese’s reduction potential through the introduction of water molecules into its structure. This development has major implications for industries burdened by the need to handle toxic hydrocarbons. By harnessing this catalyst, industries could transition toward greener processes, fundamentally changing how pollutants are treated before they enter the environment. Control over the activation of oxygen-hydrogen bonds enhances the catalyst’s performance, fostering quicker and more efficient reactions.
A Vision for the Future
Enthusiasm is warranted as we stand on the precipice of a new era in chemical engineering and environmental science. The potential to integrate such innovative catalysts into existing industrial frameworks could substantially mitigate the detrimental impacts of hydrocarbon pollution. As the world seeks effective strategies to combat pollution and advance sustainability efforts, the research led by UNIST not only exemplifies ingenuity in scientific research but also inspires a hopeful vision for a cleaner, healthier planet.
In a landscape increasingly dominated by urgency surrounding environmental issues, the advent of such technologies is not just timely—it is essential. The work of Professor Cho and his team marks a commendable stride towards harmonizing industrial processes with ecological responsibility, emphasizing that innovative chemistry can indeed lead to profound environmental transformation.

Leave a Reply