Anodes used for the electrolytic splitting of water typically rely on iridium-based materials. However, the stability of iridium catalysts in this process is a concern due to the increasing dissolution of iridium in the acidic environment of the electrolysis cell. To address this issue, researchers at HZB and HI-ERN have conducted a study to investigate the potential of titanium oxides in enhancing the stability of iridium catalysts.
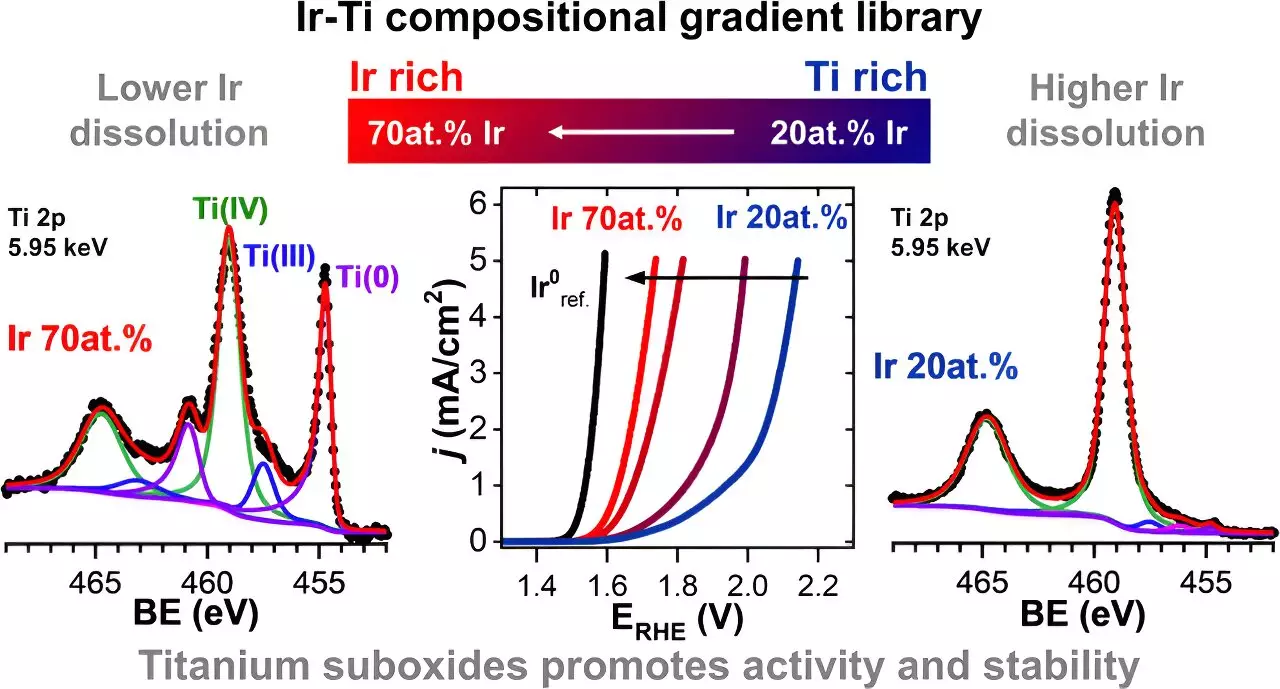
The researchers created a material library, in which they systematically varied the concentration of iridium and titanium oxides. The sample was produced at HI-ERN by sputtering titanium and iridium, resulting in a thin-film materials library with iridium content ranging from 20% to 70%. The objective was to determine the effects of different proportions of titanium oxide on the stability and catalytic activity of the iridium catalyst.
Using X-ray spectroscopic methods, the team analyzed the chemical structure of the mixed iridium-titanium oxide samples. The analysis revealed several interesting findings. Firstly, the presence of titanium suboxides, such as TiO and TiOx, improved the conductivity of the material. This finding suggests that the addition of titanium oxide can enhance the overall performance of the catalyst.
Furthermore, the researchers observed that certain titanium oxides dissolved at a faster rate in the aqueous electrolyte compared to iridium. This phenomenon led to the creation of micropores on the surface of the catalyst, facilitating the oxygen evolution reaction. The increased contact between iridium atoms from lower layers and the electrolyte contributed to the catalytic activity of the material.
Most significantly, the addition of titanium oxides, including TiO2, TiO, and TiOx, significantly reduced the dissolution of iridium. In fact, the sample with 30% titanium added exhibited an iridium dissolution approximately 70% lower compared to a pure iridium electrode material. These results indicate that the presence of titanium oxides can improve the stability and longevity of iridium catalysts.
The relevance of these findings for industry is a key consideration. While there are already established technologies and practices in place, the potential benefits of incorporating titanium oxide into iridium catalysts cannot be ignored. The improved stability of the catalysts can lead to more efficient and reliable electrolytic water splitting processes, which are crucial for the production of “green” hydrogen and energy storage from renewable sources.
However, introducing changes to established technologies is often challenging. The adoption of new materials or modifications to existing catalyst formulations requires careful evaluation and validation. The findings from this laboratory research provide valuable insights into the potential benefits of using titanium oxides in iridium catalysts, but further studies and testing are necessary to determine the scalability and practicality of these findings in an industrial setting.
The study conducted by researchers at HZB and HI-ERN offers promising insights into enhancing the stability of iridium catalysts in electrolytic water splitting. The addition of titanium oxides, as demonstrated in the material library, can have significant positive effects on the performance and longevity of iridium catalysts. Future research can build upon these findings to explore the practical applications and technical feasibility of incorporating titanium oxides into industrially relevant electrocatalytic systems. Ultimately, improving the stability of iridium catalysts can contribute to the development of more efficient and sustainable energy storage solutions.

Leave a Reply