Helices are prevalent structural motifs found in a multitude of biological molecules, particularly in proteins. These spiral shapes are not merely aesthetic; they play a vital role in determining how these molecules behave and interact within our bodies. The precise arrangement of constituent elements gives rise to a helical structure, and understanding this arrangement is paramount for unraveling the complex mechanisms at play in bodily functions. In the context of peptides—short chains of amino acids derived from proteins—their helical form results from the specific sequence and organization of these basic units.
A noteworthy aspect of helices is their chirality, an intrinsic property arising from the spatial arrangement of their components. This property is crucial as it influences how helices interact with light and other molecules, subsequently affecting their biological functions. Recent insights into this topic shed light on an additional layer of chiral information generated in alpha-helical conformations of peptides. The implications are far-reaching, providing avenues for a deeper understanding of molecular interactions that drive various biological processes.
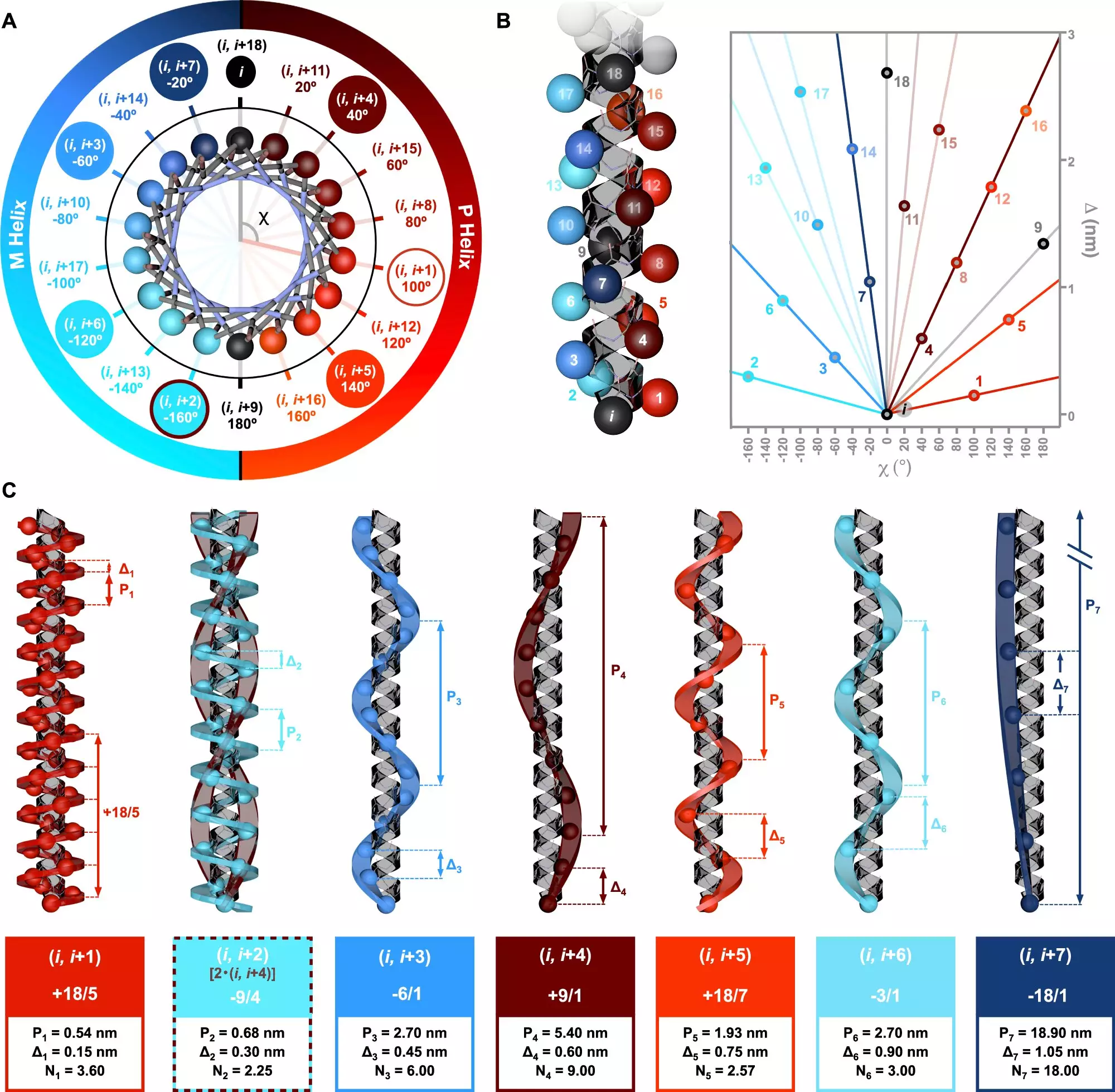
A pivotal study led by Dr. Julián Bergueiro, affiliated with the Center for Research in Biological Chemistry and Molecular Materials (CiQUS), delves into the intricacies of helices in peptides. The research, published in the respected journal *Nature Communications*, introduces a novel symmetry model that captures the interplay between the amino acid sequences and resulting helical structures. By employing advanced computational techniques alongside circularly polarized light spectroscopy, the team was able to accurately analyze how different helices form and behave when exposed to chiral light.
The findings from this research indicate a direct relationship between amino acid repetition patterns and the resultant helical shapes. This revelation is significant not only for theoretical understanding but also for practical applications in biotechnology and medicine. As Dr. Bergueiro emphasizes, this knowledge could facilitate the design of tailored molecules with specific functions—potentially transforming drug development and material science.
The ability to control the sequence of monomers alongside the architectural topology of peptide chains presents exciting possibilities in the domain of macromolecular engineering. As researchers strive to harness these molecular insights, the potential for creating innovative biocompatible materials and therapeutic agents becomes increasingly tangible. The advancements documented in this study pave the way for future explorations into peptide chemistry, promising new solutions for challenges in health and technology.
The significance of helical structures in peptide chemistry cannot be overstated. Through ongoing research and exploration, scientists stand to unlock the full potential of these fascinating molecular architectures, which may hold the key to groundbreaking discoveries in various scientific fields.


Leave a Reply