A groundbreaking study, published in the prestigious journal Chemical Science, has shed light on the remarkable properties of zirconium nitride (ZrN). This newly identified material holds the key to powering clean energy reactions, potentially revolutionizing the way we generate electricity. Researchers, led by Associate Professor Hao Li from Tohoku University’s Advanced Institute for Materials Research (WPI-AIMR), have developed a comprehensive framework that not only unravels the mysteries behind ZrN but also opens doors for future designs of transition metal nitrides. With the ability to outperform even platinum, this cost-effective alternative has the potential to transform the field of clean energy.
Anion exchange membrane fuel cells (AEMFCs) are highly promising devices that convert hydrogen and oxygen into clean electricity through chemical reactions. These reactions, namely the hydrogen oxidation reaction and the oxygen reduction reaction (ORR), have the potential to drive the transition towards a greener and more sustainable energy landscape. A noteworthy advantage of AEMFCs is their ability to operate in alkaline conditions, providing an ideal environment for catalyst materials based on earth-abundant elements. This characteristic makes them a cheaper alternative to catalysts like platinum, which are costly and scarce. However, finding efficient catalysts that can perform well in alkaline media has proven to be a challenge for scientists.
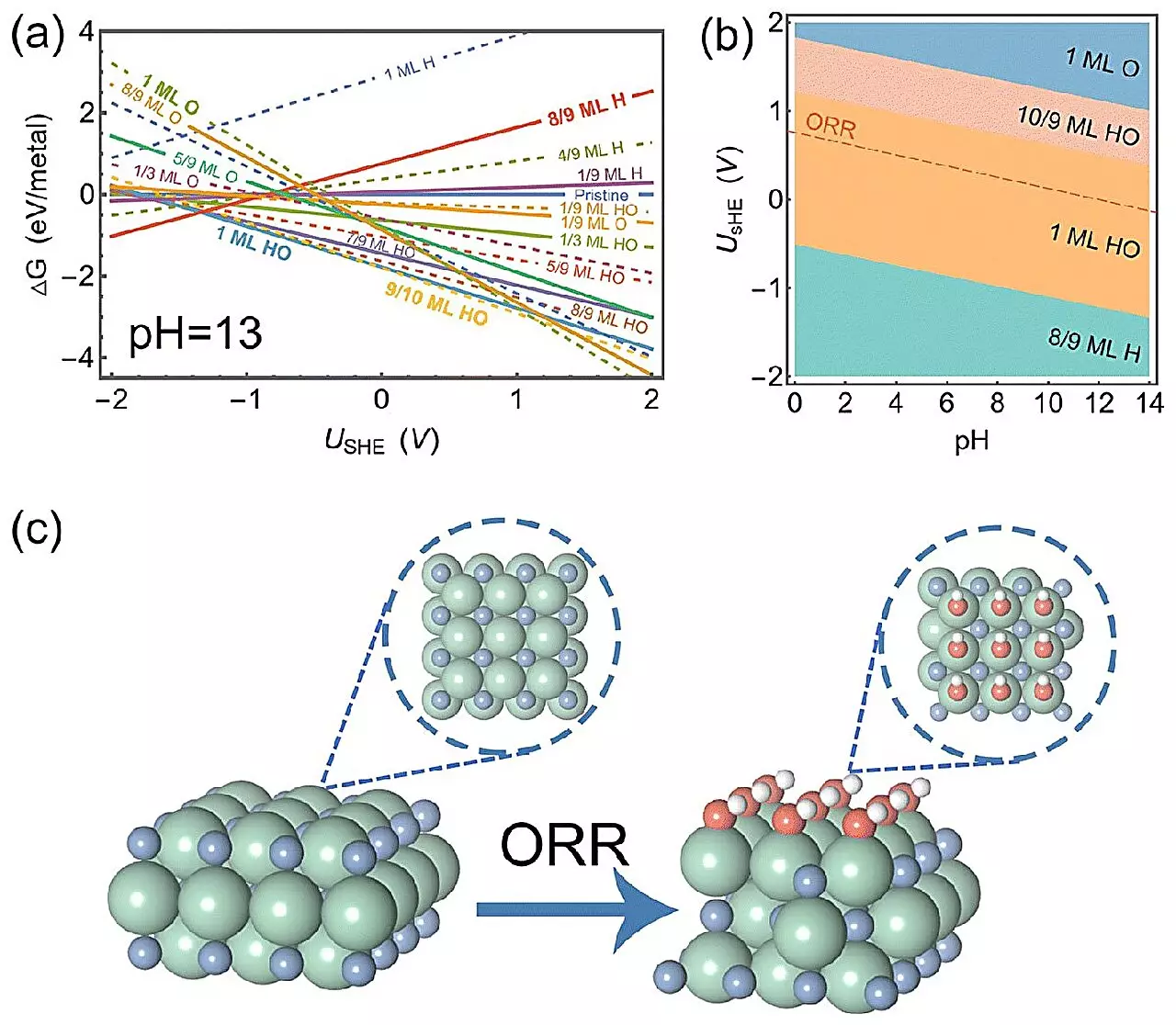
The recent studies conducted by Professor Hao Li and his team have demonstrated that ZrN exhibits remarkable efficiency in the ORR, surpassing the performance of platinum in alkaline conditions. What makes this discovery even more significant is that ZrN, though not an Earth-abundant material, is still more cost-effective compared to alternative catalysts. But what lies behind its exceptional performance has long remained a mystery.
To demystify the exceptional properties of ZrN, the researchers deployed a range of cutting-edge techniques, including surface state analysis, electric field effect simulations, and pH-dependent microkinetic modeling. Through surface analysis, they discovered that ZrN forms a thin layer of HO during the ORR process. This thin layer acts as a catalyst, providing an ideal surface for molecules to adhere to during the reaction. The electric field effect simulations further revealed that atomic oxygen, when attached to the thin-covered surface, undergoes minimal changes, allowing it to stick moderately. These findings highlight the unique characteristics of ZrN that enable it to reach the optimal conditions for ORR in alkaline environments.
Significantly, the researchers’ theoretical framework for ZrN goes beyond this specific material. It has been successfully applied to other transition metal nitrides like Fe3N, TiN, and HfN, which share similar properties with ZrN. This universality provides a broader understanding of how these materials can be utilized for clean energy applications. By rationalizing and designing transition metal nitrides for alkaline ORR, this framework has the potential to guide the development of more efficient catalyst materials in the future.
Looking ahead, Professor Hao Li and his team plan to extend their framework to investigate other industrially significant reactions, such as the oxygen evolution reaction. By gaining deeper insights into these reactions, they aim to further advance the field of clean energy and contribute to the global efforts for a sustainable future.
The study on ZrN marks a significant step forward in the quest for cleaner energy. The unraveled mysteries surrounding this material and the comprehensive framework developed by Professor Hao Li and his team hold immense potential for revolutionizing catalyst materials used in energy conversion reactions. With the promise of cost-effectiveness and superior performance, ZrN is paving the way for a greener and more sustainable energy landscape.


Leave a Reply