The persistent rise in atmospheric CO2 levels necessitates the exploration of innovative methods for its utilization. Among these methods, the electrochemical reduction of CO2 has emerged as a particularly promising strategy. Traditionally, research has focused heavily on the design and improvement of catalysts to enhance the selectivity of reaction products. However, a critical, yet often overlooked, factor influencing the selectivity of these products is the composition of the electrolyte used during the reduction process. This article explores new research findings on a metal-organic framework (MOF) electrocatalyst that emphasizes the importance of electrolyte composition in shaping the outcomes of CO2 reduction.
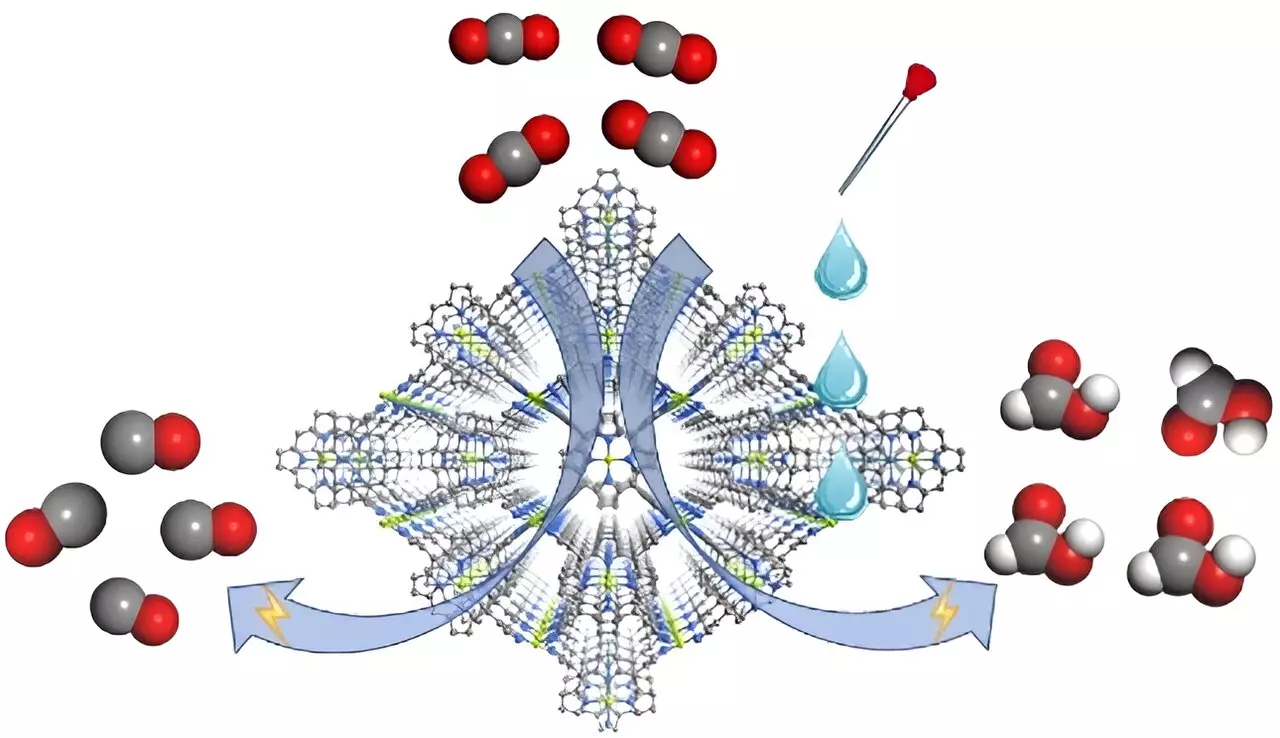
Recent work published in Angewandte Chemie International Edition introduces a novel MOF electrocatalyst named FICN-8, engineered by a team of researchers, including prominent figures such as Prof. Cao Rong and Prof. Zhang Teng. This catalyst comprises Cu-based components and has a carefully constructed three-dimensional porous architecture that significantly enhances substrate accessibility to the active metalloporphyrin sites. The research team was able to leverage this unique structure to run experiments under various solvent and electrolyte conditions, paving the way for a deeper understanding of how electrolyte interactions affect the selectivity of CO2 reduction products.
One of the breakthrough findings of this research stems from the tuning of electrolyte composition, which was shown to dramatically influence the product selectivity of CO2 reduction. During electrochemical testing in a specific electrolyte mixture containing tetrabutylammonium hexafluorophosphate (TBAPF6) and acetonitrile, the FICN-8 catalyst exhibited an impressive 95% selectivity towards the production of carbon monoxide (CO). However, the introduction of water or trifluoroethanol (TFE) into the electrolyte yielded a stark shift in product formation from CO to formic acid, with maximum Faradaic efficiencies reaching 48% under certain conditions.
The researchers employed a series of experiments to elucidate the mechanisms behind this switch in selectivity. Through kinetic isotope effect (KIE) measurements, valuable insights were gained regarding the involvement of protons in the formation of formic acid, indicating a more complex reaction pathway compared to that of CO production. The theoretical calculations conducted further identified the adsorption of hydride (*H) on the porphyrin nitrogen site as pivotal to the formation of formic acid, indicating a dual-pathway mechanism dependent on the concentration of protons in the system.
This research underscores the critical role that electrolyte composition plays in the electrochemical reduction of CO2, shifting the focus towards optimizing not only catalysts but also the electrolyte systems utilized. The findings propel us toward a new horizon in designed catalyst-electrolyte systems aimed at achieving higher selectivity for value-added products from CO2. Future work in this domain will potentially unlock further advancements, allowing for more effective and selective strategies in contemporary carbon capture and utilization technologies.
This study paves the way for future innovations in electrochemical CO2 reduction, emphasizing that a holistic approach—including the chemical environment of the electrolyte—could refine the pathways towards sustainable carbon utilization. The implications of this research stretch beyond theoretical understandings, calling for practical applications in real-world energy solutions.


Leave a Reply