Oregon State University researchers have made a groundbreaking discovery in the field of clean energy. Led by Kyriakos Stylianou from the OSU College of Science, a team of scientists has developed a new material with the potential to transform sunlight and water into clean energy at an unprecedented speed and efficiency.
Stylianou’s research has centered around metal organic frameworks (MOFs), crystalline, porous materials with unique properties. These MOFs consist of positively charged metal ions surrounded by organic “linker” molecules, creating nanosized pores with adjustable structural characteristics. By designing MOFs with specific components, researchers can tailor the properties of these materials for various applications.
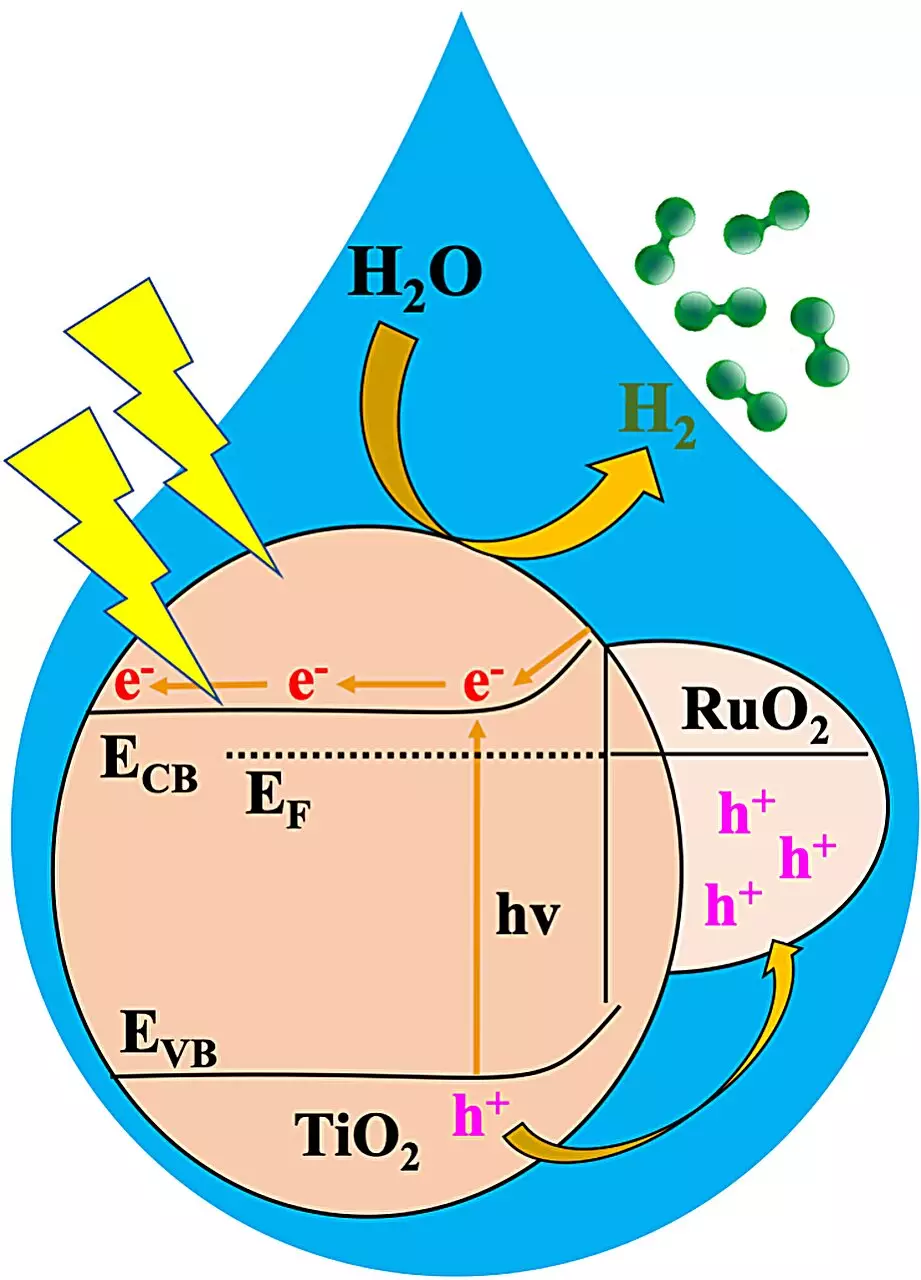
In their recent study published in Angewandte Chemie, the team utilized a MOF to develop a metal oxide heterojunction known as RTTA. This innovative catalyst, composed of ruthenium oxide and titanium oxide doped with sulfur and nitrogen, demonstrated exceptional performance in splitting water into hydrogen when exposed to sunlight. Among multiple RTTA variations tested, RTTA-1 emerged as the most efficient, producing over 10,700 micromoles of hydrogen in just one hour.
One of the most impressive findings of the study was the quantum yield of RTTA-1, which reached 10%. This means that for every 100 photons striking RTTA-1, 10 photons contributed to hydrogen production, showcasing the remarkable efficiency of this photocatalyst. Stylianou attributes the superior performance of RTTA-1 to the synergistic effects of the metal oxides’ properties and surface characteristics derived from the parent MOF.
The development of MOF-derived metal oxide heterojunctions like RTTA-1 opens up new possibilities for practical hydrogen production. By utilizing photocatalysis to split water and generate hydrogen, researchers are paving the way for sustainable and efficient energy solutions that could combat greenhouse gas emissions and climate change.
Compared to traditional methods of hydrogen production, such as methane-steam reforming, the catalytic process of splitting water through photocatalysis offers a cleaner and more sustainable alternative. While current electrocatalytic processes require electricity, the photocatalytic approach leverages the Earth’s abundant solar energy to produce hydrogen from water. Despite the higher cost of materials like ruthenium oxide, the minimal amount used in the photocatalyst makes it a viable and cost-effective solution for green hydrogen production.
The research conducted by Oregon State University researchers represents a significant step forward in the quest for renewable and clean energy sources. By harnessing the power of sunlight and water through innovative materials like MOF-derived metal oxide heterojunctions, scientists are opening doors to a more sustainable future. The potential of these technologies to revolutionize hydrogen production and reduce reliance on fossil fuels highlights the importance of continued research and investment in alternative energy sources.

Leave a Reply