The field of cryo-electron tomography (cryo-ET) has revolutionized the way scientists visualize and analyze cellular structures within their natural environment. This cutting-edge technology has opened up new avenues for studying complex biological processes at the molecular level. In a recent study conducted by researchers at the MPI of Biochemistry in Martinsried and the University Medical Center Göttingen, cryo-ET was employed to investigate protein folding helpers known as chaperonin complexes in the bacterium E. coli. The findings of this study, published in the prestigious journal Nature, shed light on the intricate mechanisms underlying protein folding and have far-reaching implications for understanding diseases such as Alzheimer’s and Parkinson’s.
Proteins are the building blocks of life, responsible for carrying out a myriad of functions within cells. However, in order to function properly, proteins must adopt specific three-dimensional structures. Chaperonins, specialized protein folding assistants, play a crucial role in ensuring that newly synthesized proteins achieve their correct conformation. These chaperonin complexes, consisting of subunits GroEL and GroES in bacteria, provide a protective environment for proteins to fold correctly, shielding them from the chaotic cellular milieu. Understanding the structure and function of chaperonins is essential for unraveling the mysteries of protein folding and developing targeted therapies for protein misfolding diseases.
Biochemist F.-Ulrich Hartl, a pioneer in the field of chaperonins, joined forces with structural biologists Wolfgang Baumeister and Rubén Fernández-Busnadiego to delve deeper into the workings of these intricate protein folding machines. Through a combination of cryo-ET, single-particle cryo-electron microscopy (cryo-EM), and quantitative mass spectrometry, the researchers were able to capture unprecedented snapshots of chaperonin complexes in action within living cells. This innovative approach marked a significant advancement in the field, enabling the direct visualization of chaperonins within their native cellular environment, which was previously unattainable due to the complex and dynamic nature of these molecular machines.
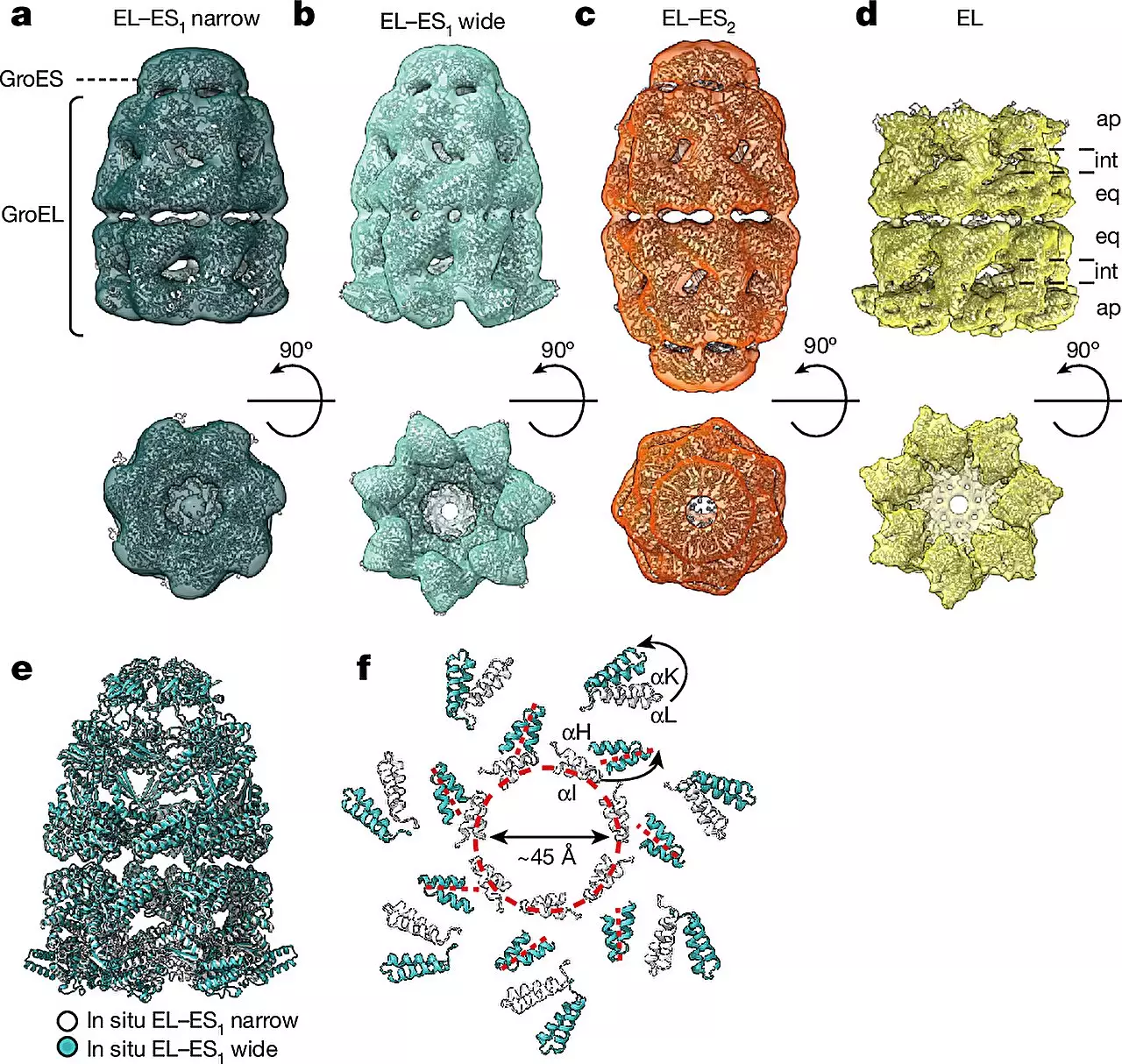
The study revealed two distinct forms of the GroEL-GroES chaperonin complex, aptly named “bullet” and “football” due to their structural symmetries. The bullet form, characterized by a GroES cap bound to one side of the GroEL barrel, was predominantly observed in bacteria during normal growth conditions. In contrast, the football complexes displayed a different structural arrangement, offering new insights into the flexibility and adaptability of chaperonins during the protein folding process. By observing these complexes at high resolution in their native cellular context, the researchers were able to unravel the intricate dance of chaperonins as they facilitate protein folding, shedding light on their dynamic assembly and conformational changes.
The implications of this research extend beyond the realm of basic science, with potential implications for the development of novel therapeutic strategies for protein misfolding diseases. By gaining a deeper understanding of how chaperonins operate and adapt to different cellular conditions, researchers can pave the way for targeted interventions to correct protein misfolding and prevent the onset of devastating neurodegenerative disorders. The insights gained from studying chaperonin complexes using cryo-ET represent a significant leap forward in our quest to decipher the intricacies of protein folding and its implications for human health.
The integration of cryo-ET with cutting-edge imaging techniques has opened up new vistas for exploring the complex world of protein folding helpers. By peering into the molecular machinery of chaperonins within living cells, researchers are uncovering a treasure trove of information that could revolutionize our approach to treating protein misfolding diseases. This study serves as a testament to the power of interdisciplinary collaboration and innovative technologies in unraveling the mysteries of life at the molecular level.

Leave a Reply