In the world of organic chemistry, breakthroughs are rare and far between. However, a recent study has shed light on a new method that could potentially revolutionize the way we synthesize complex molecules. The anti-Michael addition reaction, which has long been elusive due to the higher electrophilicity of the β-position, has finally been achieved by a global team of researchers led by Professor Takanori Matsuda from Tokyo University of Science. This groundbreaking discovery opens up new possibilities for the synthesis of α-substituted carbonyl compounds, which are essential building blocks in the pharmaceutical industry.
Traditionally, the Michael addition reaction has been extensively studied and utilized in organic synthesis. However, achieving the reverse reaction, the anti-Michael addition, has proven to be a challenging task. Previous attempts to overcome this difficulty involved intramolecular reactions or the introduction of strong electron-withdrawing groups at the β-position. While these methods have shown some success, they are not ideal for synthesizing complex molecules. The breakthrough in the anti-Michael addition reaction brings new hope for chemists around the world.
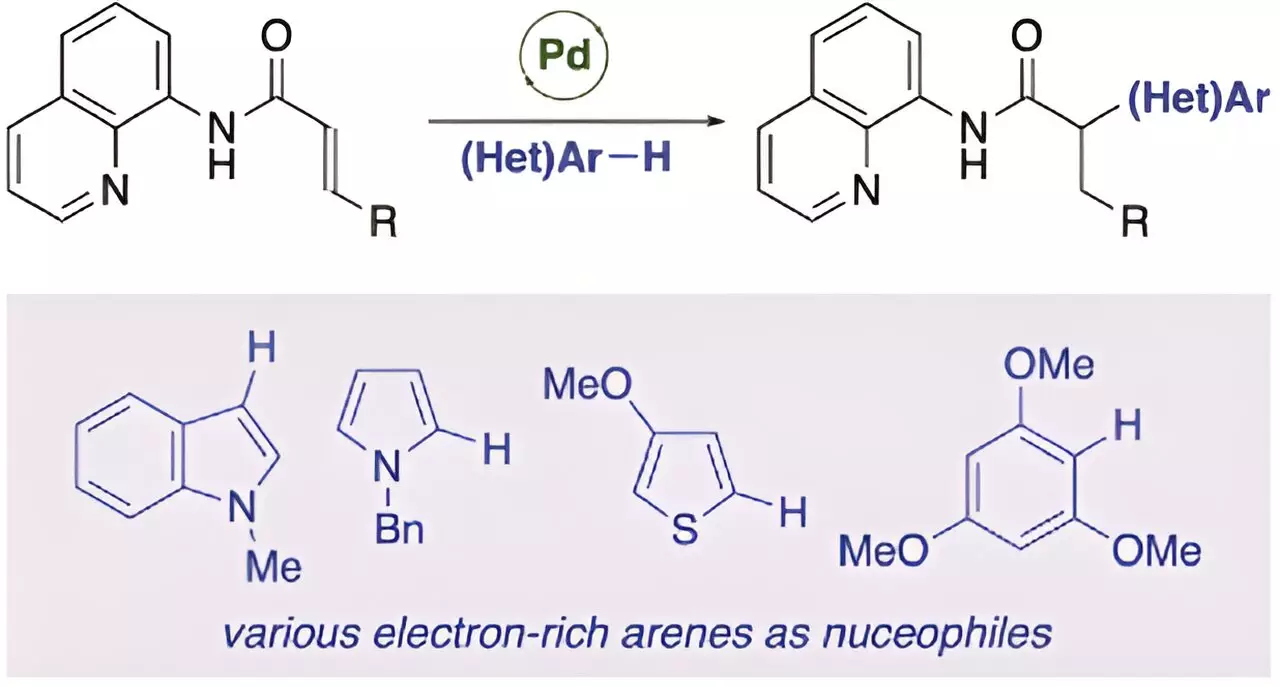
In their study, Professor Matsuda and his team successfully achieved the palladium-catalyzed anti-Michael addition reaction of acrylamides. This marks the first example of an anti-Michael-type addition reaction. By introducing a directing group into the α,β-unsaturated carbonyl compound, the researchers were able to stabilize the reaction intermediate and facilitate the anti-Michael type addition reaction. The presence of a catalytic amount of palladium(II) trifluoroacetate was crucial in achieving high yields of the addition product.
Through labeling experiments, the researchers were able to elucidate the mechanism behind the anti-Michael addition reaction. The reaction proceeds with the formation of a five-membered ring intermediate, followed by the nucleophilic attack and the regeneration of the palladium catalyst. This efficient mechanism opens up a wide range of possibilities for the synthesis of α-substituted carbonyl compounds using a variety of nucleophiles.
The discovery of the anti-Michael addition reaction represents a significant milestone in the field of organic chemistry. This novel method offers a more efficient and sustainable way to synthesize complex molecules, particularly α-substituted carbonyl compounds that are essential in drug development. The potential applications of this study are vast, and it is expected to have a lasting impact on the future of organic synthesis. Dr. Suzuki emphasizes that this new reaction could become an ideal one-step process with 100% atomic efficiency, paving the way for the widespread application of this revolutionary method.


Leave a Reply