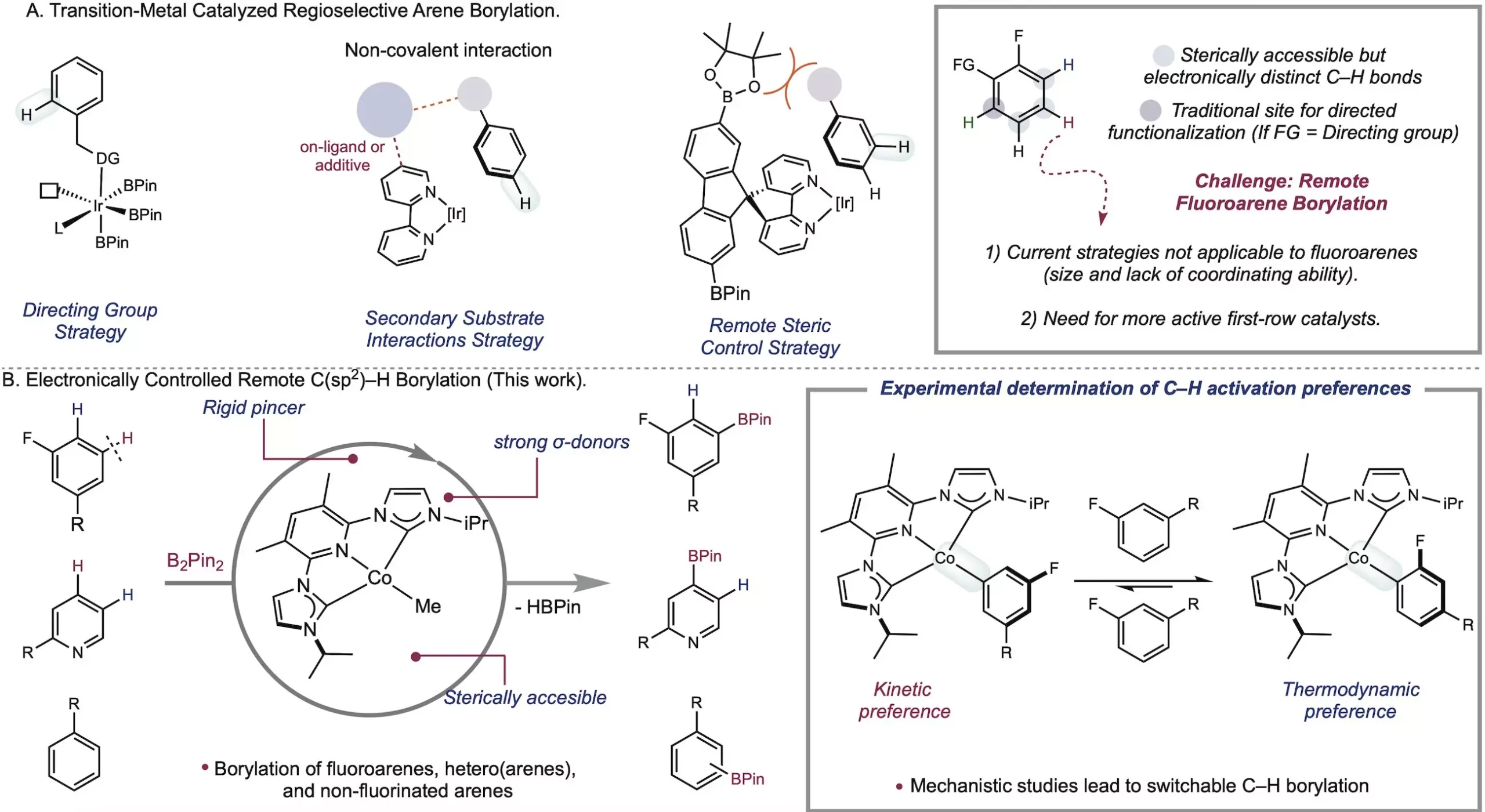
In the field of metal-catalyzed C–H functionalization, the Chirik Group at the Princeton Department of Chemistry has been making significant strides. Their latest breakthrough involves the use of a cobalt catalyst that can differentiate between bonds in fluoroarenes based on their intrinsic electronic properties, allowing for meta-selective borylation. This groundbreaking research, published in Science, showcases the Chirik Lab’s innovative approach in understanding and manipulating organometallic chemistry. With the potential to revolutionize the synthesis of medicines, natural products, and materials, this method holds promise for various industries.
Traditionally, chemists have relied on conventional methods to functionalize benzene rings. However, the Chirik Group has been focused on developing new avenues for exploration. Lead author Jose Roque, a former postdoc in the Chirik Group, successfully achieved meta-selective C–H borylation using a cobalt catalyst, a feat previously unaccomplished. This breakthrough is significant as meta C–H bonds are notoriously challenging to selectively functionalize. By creating a cobalt catalyst that is both fast and selective, the Chirik Group has opened doors to exciting possibilities.
The success of the Chirik Group’s research can be attributed to their rational design approach. Early stoichiometric studies indicated high activity for C–H activation, prompting the lab to pursue a catalyst that would lead to even greater reactivity. By designing an electronically rich but sterically accessible pincer ligand, the Chirik Group hypothesized that they could create a more active cobalt catalyst. Their hypothesis proved correct, resulting in an optimized catalyst that exhibits exceptional reactivity.
While iridium has long been the catalyst of choice for sterically driven C–H functionalization, its inability to selectively target desired bonds in molecules with multiple C–H bonds presented a significant challenge. This limitation prompted pharmaceutical companies to seek alternatives with greater selectivity. The Chirik Lab has focused on exploring first-row transition metals, such as cobalt and iron, which are more affordable and sustainable than iridium. Since their initial work on C–H borylation in 2014, the Chirik Lab has been at the forefront of electronically controlled C–H activation. By exploiting the differences in metal-carbon bond strength, chemists can selectively target specific bonds and achieve desired functionalization. Additionally, the Chirik Lab discovered that site selectivity can be switched by harnessing the kinetic or thermodynamic preferences of C–H activation, providing a streamlined and cost-effective process.
This research opens up new possibilities for C–H functionalization by considering metal-carbon bond strengths and predicting reaction outcomes. The selective functionalization of meta-to-fluorine bonds, which was previously challenging, has been significantly advanced through this research. Moreover, the Chirik Group has expanded their methodology to include other substrate classes beyond fluoroarenes, broadening the scope of application. With cobalt catalysts, chemists can achieve what iridium cannot, unlocking a wealth of opportunities in the field of C–H functionalization.
While the Chirik Group’s breakthrough has already made a significant impact, their work is far from over. Postdoc Alex Shimozono, who joined the project later, will play a crucial role in further advancing borylation techniques. The innovative catalyst developed by Jose Roque has already challenged the notion that a completely different catalyst is needed to change site selectivity. Shimozono’s continued research aims to push the boundaries of selective borylation and expand the arsenal of methods available to chemists.
The research conducted by the Chirik Group at the Princeton Department of Chemistry represents a significant breakthrough in the field of C–H functionalization. By using cobalt catalysts to selectively target bonds based on their intrinsic electronic properties, the group has overcome traditional limitations and opened up new possibilities for drug discovery, materials synthesis, and more. This innovative approach, driven by deep insights into organometallic chemistry, has the potential to reshape the landscape of synthetic chemistry and pave the way for groundbreaking discoveries in various industries. The Chirik Group’s research serves as a testament to the power of rational design and the importance of pushing boundaries in scientific exploration.


Leave a Reply