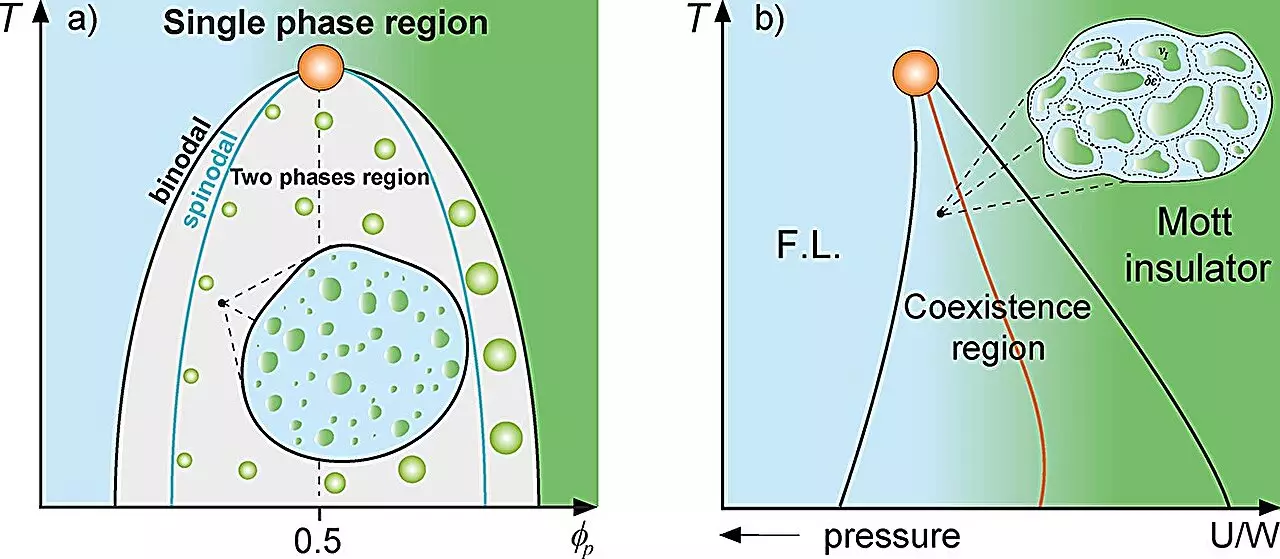
Recent advancements in condensed matter physics have shed light on biological systems, particularly regarding the behavior of proteins within cells. Traditionally, mixture theory in physics helps describe systems made up of multiple constituents, focusing on their respective fractions and interactions. This theoretical framework is essential to understanding phenomena such as the coexistence of different states in supercooled water and the Mott metal-insulator transition, where metal puddles exist in an insulating framework.
Research conducted by a team from São Paulo State University (UNESP) introduces intriguing parallels between these physical principles and protein compartmentalization within cells. By employing theories from condensed matter physics, the researchers propose a novel concept—the cellular Griffiths-like phase. This concept draws an analogy with the magnetic Griffiths phase observed in materials science, where regions with distinct magnetization emerge in a general paramagnetic or ferromagnetic medium. The implications of these findings extend well beyond pure science, suggesting a deeper connection to the origins of life itself.
According to the research, within the cellular environment, proteins can spontaneously undergo liquid-liquid phase separation, leading to the formation of protein-rich droplets. This phenomenon resonates with the behavior of “rare regions” seen in magnetic systems, where dynamics are substantially slowed down due to the random emergence of these regions. The principal investigator, Mariano de Souza, emphasizes how this cellular dynamics reduction occurs near specific thresholds, analogous to the binodal line that demarcates phase separation.
Utilizing a variety of thermodynamic models, including the Grüneisen parameter and the Flory-Huggins model, the researchers were able to illustrate that the dynamics of proteins are significantly impaired in certain conditions. They argue that this slowing of dynamics could be pivotal in understanding the fundamental conditions necessary for life to evolve. The study additionally suggests that these Griffiths-like cellular phases might have played a vital role during the origin of life, reflecting on the classical concepts put forth by biochemist Aleksandr Oparin in the 1930s.
A fascinating aspect of this research is its incorporation of chirality—the property of molecules that distinguishes their mirror-image forms. Homochirality, which refers to the dominance of one chiral form over another in biological systems, is argued to have been crucial for the emergence and evolution of life. The study aligns chiral properties with cellular dynamics, highlighting that coacervates—droplets formed from organic molecules—might have originated under conditions of reduced dynamics, allowing for evolutionary advantages.
In this context, the research posits that increased protein diffusion times coincide with diminished stochastic fluctuations, thus optimizing gene expression. This insight offers a novel lens through which to examine how life might have transitioned from simple organic molecules to more complex cellular forms.
Broader Implications in Health and Disease
The relevance of liquid-liquid phase separation extends into medicine and health sciences, especially concerning diseases linked to protein malfunction. The interplay between phase separation and various pathologies, including cancer and neurodegenerative diseases, is gaining attention. For instance, the compartmentalization of disease-linked proteins could obstruct the processes leading to cell mutation and tumorigenesis, as noted by co-author Marcos Minicucci.
As diseases like COVID-19 demonstrate, the inability of immune responses to combat viral agents can also be tied to protein coacervation. The FSP1 protein’s phase separation, for instance, presents novel therapeutic opportunities against cancer, indicating that understanding these dynamics could pave the way for innovative treatment strategies.
The research spearheaded by de Souza underscores the need for an interdisciplinary approach in scientific inquiry. The collaboration between physicists, biologists, and medical experts highlights how blending different fields can yield insights that would be unattainable in isolation. The significance of the findings extends to the broader scientific community, suggesting that the laws governing physical systems may have far-reaching consequences for understanding biological phenomena and the evolution of life itself.
The Griffiths-like cellular phase proposed in this study not only offers a fresh perspective on protein behavior but also serves as a reminder of the intricate connections between physical systems, biological processes, and evolutionary mechanisms. As such, this research opens the door to new explorations at the intersection of the physical and biological sciences, potentially transforming our approach to both fundamental research and practical health applications.


Leave a Reply