In a recent study conducted by researchers at the University of Liverpool, a groundbreaking discovery has been made in the realm of sustainable fuel production. The team has successfully developed a plasma-catalytic process for the conversion of carbon dioxide (CO2) into methanol at room temperature and atmospheric pressure. This innovative approach addresses the inherent limitations of traditional thermal catalysis, which typically requires high temperatures and pressures, resulting in low CO2 conversion rates and methanol yields.
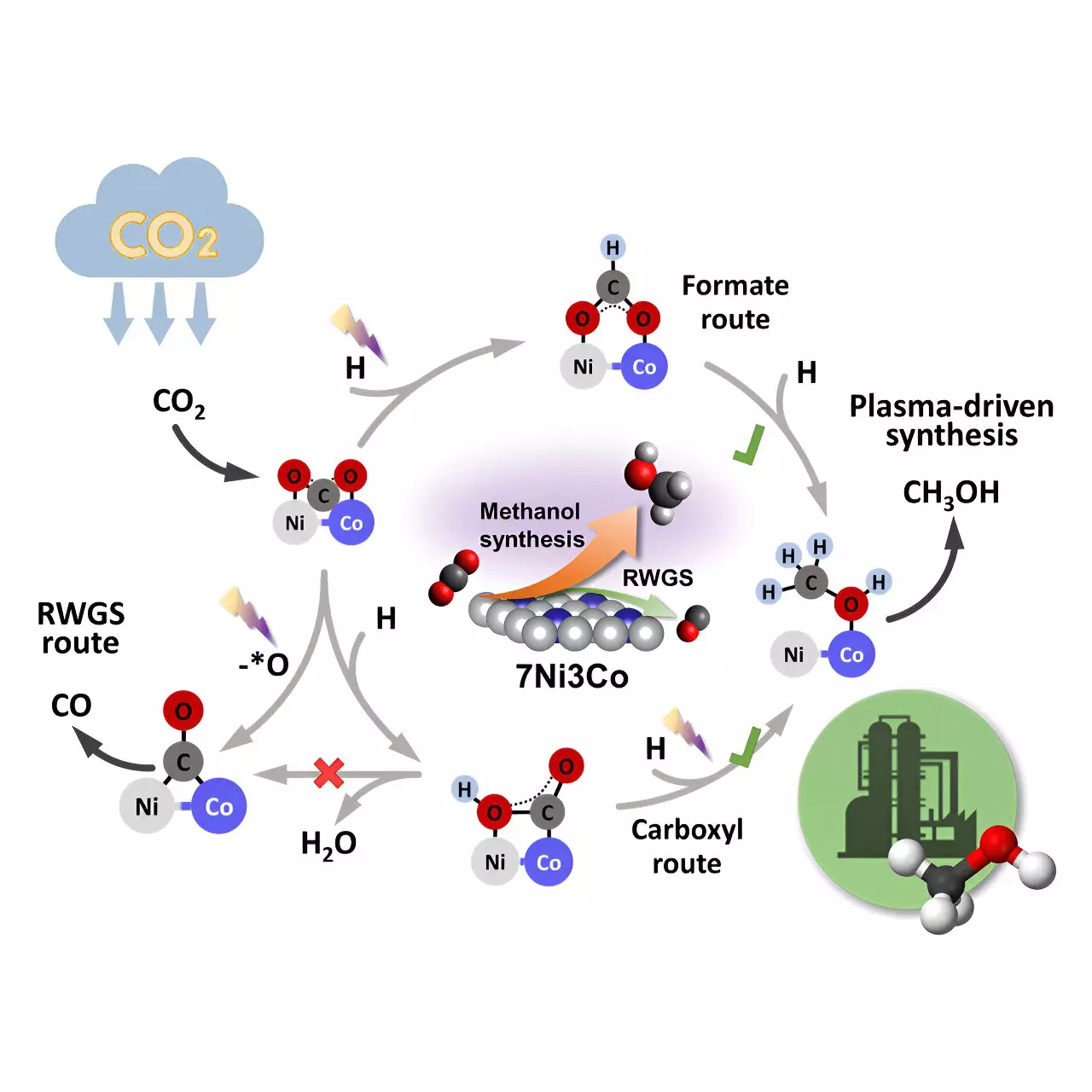
Non-thermal plasma, an ionized gas containing energetic electrons and reactive species, plays a crucial role in this process by activating strong chemical bonds of inert molecules like CO2. This activation facilitates chemical reactions under mild conditions, allowing for the conversion of CO2 to methanol with impressive selectivity and efficiency. The use of a bimetallic Ni-Co catalyst within a non-thermal plasma reactor has enabled the researchers to achieve a single-pass 46% selectivity for methanol and 24% CO2 conversion at 35°C and 0.1 MPa.
In situ plasma-coupled Fourier transform infrared (FTIR) characterization and density functional theory (DFT) calculations have revealed that the bimetallic Ni-Co interface serves as the primary active center for methanol synthesis. The Eley-Rideal (E-R) mechanism facilitates CO2 adsorption and hydrogenation, leading to the production of various intermediates crucial for methanol formation. By controlling the Ni-Co sites in bimetallic catalysts, researchers can tailor the weight of each reaction pathway, promoting asymmetric adsorption of CO2 molecules at the bimetallic interfaces to modulate product distribution effectively.
This research showcases the significant potential of plasma catalysis as an emerging electrification technology for sustainable CO2 conversion and fuel production. The ability to conduct these reactions at ambient conditions using a modular and scalable plasma system presents a compelling alternative for the chemical industry. Moreover, plasma-based systems can be powered by intermittent renewable electricity, further enhancing the feasibility of decentralized fuel and chemical production.
The University of Liverpool research team’s pioneering work represents a major step forward in the field of catalytic CO2 conversion. Their findings not only offer promising avenues for future research but also hold tremendous potential for industrial applications aimed at achieving a sustainable future. With a focus on plasma catalysis, the team continues to explore innovative methods for converting CO2 into valuable fuels and chemicals, demonstrating a commitment to advancing sustainable fuel production technologies.

Leave a Reply