When you consider a battery, the image of stretchy material does not likely come to mind. However, as flexible electronics become increasingly popular for wearable health monitors, batteries with a shape-shifting quality are essential. Recent research published in ACS Energy Letters introduces a lithium-ion battery with entirely stretchable components. This groundbreaking battery includes an electrolyte layer that can expand by an impressive 5,000%. Additionally, it retains its charge storage capacity after nearly 70 charge/discharge cycles. The demand for batteries that can bend and stretch in tandem with flexible electronics is on the rise.
Traditionally, researchers have attempted to construct stretchable batteries using woven conductive fabric or rigid components folded into expandable shapes reminiscent of origami. However, achieving a truly malleable battery requires all parts, including the electrodes that collect charge and the middle electrolyte layer for charge balance, to be elastic. Existing stretchy battery prototypes often exhibit moderate elasticity, involve complex assembly processes, or have limited energy storage capacity, especially over time with repeated charging and discharging. One common issue is the weak connection between the electrolyte layer and the electrodes, or the instability of the fluid electrolyte, resulting in movement when the battery changes shape.
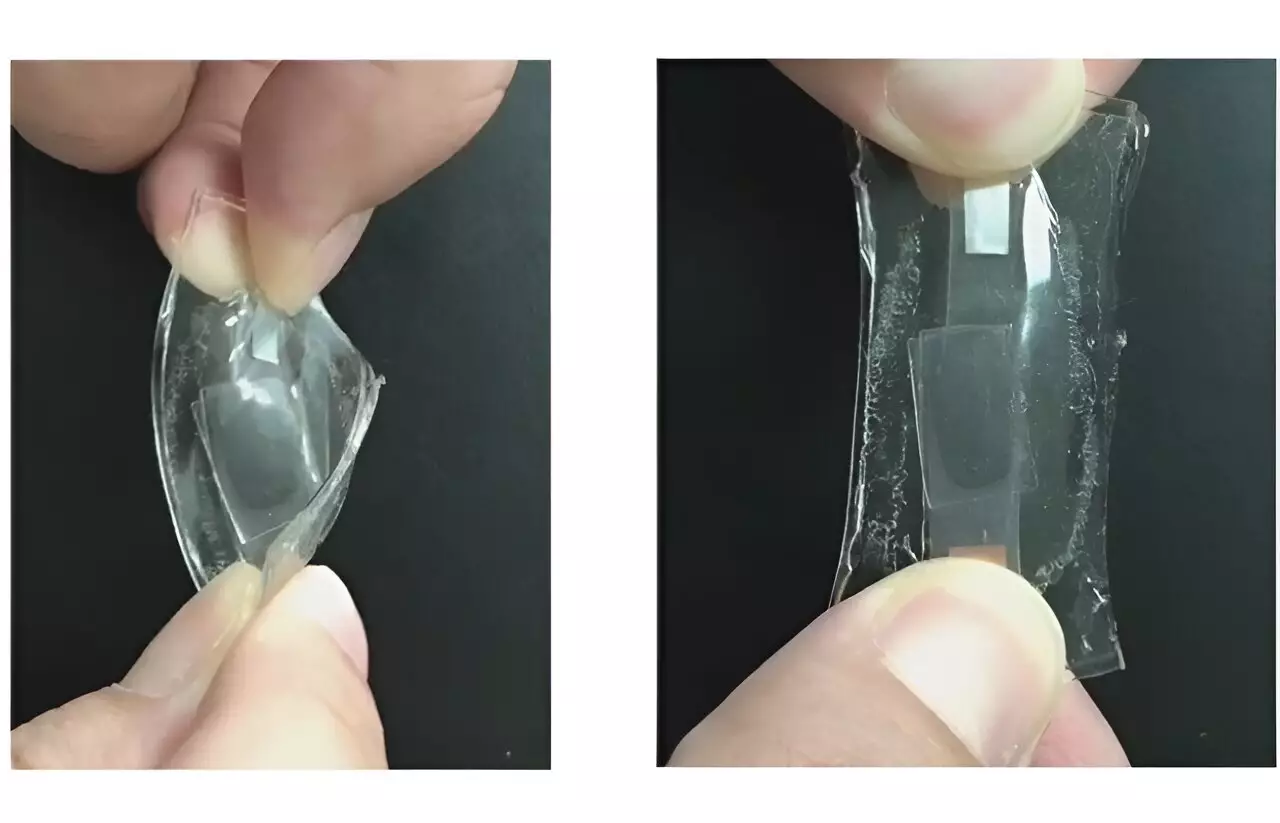
In an effort to address these challenges, researchers led by Wen-Yong Lai opted for a different path. Instead of using a liquid electrolyte, they aimed to integrate the electrolyte into a polymer layer sandwiched between two flexible electrode films to create a solid, stretchy battery. To fabricate the electrodes for this fully elastic battery, the team applied a thin film of conductive paste containing silver nanowires, carbon black, and lithium-based materials onto a plate. A layer of polydimethylsiloxane, a flexible material commonly found in contact lenses, was then added atop the paste. Subsequently, the researchers introduced a lithium salt, a highly conductive liquid, and components for a stretchy polymer directly onto this film. Upon activation by light, these elements combined to form a solid, rubbery layer capable of stretching to an astonishing 5,000% of its original length while facilitating the transport of lithium ions. The final step involved placing another electrode film on top of the stack and sealing the entire device in a protective coating.
The solid stretchy battery design demonstrated superior performance compared to similar devices using traditional liquid electrolytes. The new version exhibited an approximately sixfold increase in average charge capacity at a fast-charging rate. Moreover, the solid battery maintained a more stable capacity throughout 67 charging and discharging cycles. In contrast, other prototypes featuring solid electrodes showed steady operation over 1,000 cycles, with a mere 1% drop in capacity during the initial 30 cycles, as opposed to a significant 16% decrease for the liquid electrolyte. While enhancements are still required, this innovative approach to creating fully stretchable, solid batteries could mark a significant advancement for wearable or implantable devices designed to move and flex seamlessly with the human body.

Leave a Reply