Perfluoroalkyl substances (PFAS), commonly referred to as “forever chemicals,” have emerged as a significant environmental and health concern due to their widespread use and persistence. Initially embraced for their exceptional stability and resistance to water and heat, PFAS have found applications in a wide range of products, including cookware, clothing, and firefighting foam. However, their longevity in the environment has led to their accumulation in water, soil, and even human bodies, where they are known to induce carcinogenic and hormonal effects.
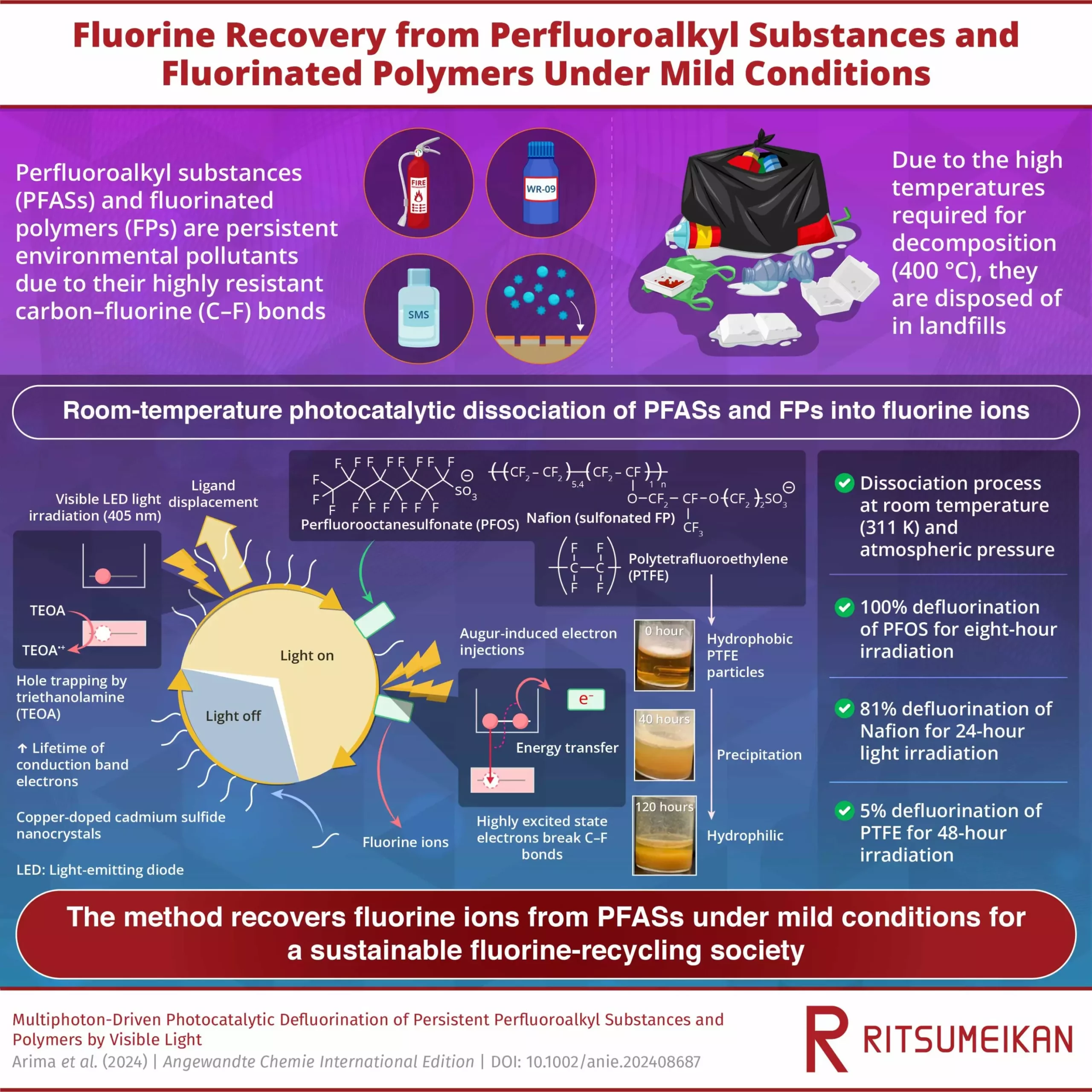
Despite efforts to phase out the production of PFAS, the challenge lies in their decomposition, which requires temperatures exceeding 400°C. As a result, products containing PFAS end up in landfills, posing potential contamination risks for the future. The inability to effectively treat PFAS has been a persistent obstacle in environmental preservation efforts.
Researchers at Ritsumeikan University have proposed a groundbreaking room-temperature defluorination method that could revolutionize the treatment of PFAS. Their innovative photocatalytic approach, detailed in a study published in Angewandte Chemie International Edition, utilizes visible light to break down PFAS and other fluorinated polymers (FPs) into fluorine ions under gentle conditions. This method offers a promising avenue for the sustainable decomposition of diverse PFAS substances.
The researchers irradiated cadmium sulfide (CdS) nanocrystals and copper-doped CdS (Cu-CdS) nanocrystals with visible LED light in the presence of perfluoroalkyl substances, fluorinated polymers, and triethanolamine (TEOA). This photocatalytic reaction generated electrons with high reduction potential, enabling the breakdown of the robust carbon-fluorine bonds in PFAS molecules. By exciting the semiconductor nanocrystals, the researchers created a conducive environment for PFAS molecules to adsorb onto the surface of the nanoparticles.
Through their method, the researchers achieved remarkable defluorination efficiencies, with 100% defluorination of perfluorooctanesulfonate (PFOS) within just 8 hours of light exposure. The defluorination efficiency increased with the duration of light irradiation, showcasing the effectiveness of the room-temperature defluorination method. Additionally, the researchers successfully defluorinated Nafion, a widely used fluoropolymer, demonstrating the versatility of their approach in treating different fluorinated compounds.
By recovering fluorine from waste PFAS using this innovative technique, the researchers have opened up possibilities for reducing reliance on fluorine production and establishing a more sustainable recycling process. This breakthrough holds significant implications for various industries that heavily rely on fluorine, including pharmaceuticals and clean energy technologies. By contributing to the development of recycling technologies for fluorine elements, this method aligns with the goals of building a more sustainable and prosperous society.
The room-temperature defluorination method proposed by researchers at Ritsumeikan University represents a critical advancement in the treatment of PFAS and fluorinated polymers. By offering a sustainable and efficient approach to decomposing these persistent chemicals, this innovative technique paves the way for enhanced environmental preservation and resource recovery. With further research and development, this method has the potential to redefine the future of PFAS treatment and contribute to a more sustainable and eco-conscious society.

Leave a Reply