The field of battery technology is constantly evolving, with researchers continually striving to develop new and improved energy storage solutions. One area of particular interest is lithium-metal batteries, which have the potential to offer significantly higher energy densities than the lithium-ion batteries that currently dominate the market. However, lithium-metal batteries have historically been plagued by limitations, most notably a short lifespan. In a recent study published in Nature Energy, researchers at the University of Science and Technology of China introduced a new electrolyte design that could address these limitations and pave the way for the development of highly performing lithium-metal pouch cells with extended lifespans.
Lithium-metal batteries have long been considered the “holy grail” of battery technology due to their ultra-high energy density. However, existing lithium-metal cells have been limited by a cycle life of only around 50 cycles, far below the approximately 1,000 cycles that commercial lithium-ion batteries can achieve. The primary factors contributing to this lower lifespan are the growth of lithium dendrites, the reactivity of lithium-metal, and the high-voltage transition metal cathodes, all of which lead to the continuous degradation of the electrolyte. Despite efforts by researchers around the world, achieving the desired energy density of over 500 Wh/kg with 1,000 cycles has remained a significant challenge.
Approximately five years ago, Prof. Shuhong Jiao and her colleagues developed an electrolyte that effectively stabilizes both the anode-electrolyte and cathode-electrolyte interfaces in lithium-metal battery cells, thereby suppressing electrolyte degradation. Building on previous research into the microscopic processes within lithium-metal batteries, the researchers focused on designing an electrolyte that could enhance battery performance using cost-effective components. By collaborating with other teams capable of theoretical calculations and microscopic characterization, they were able to develop a new class of electrolytes with a unique solvation structure.
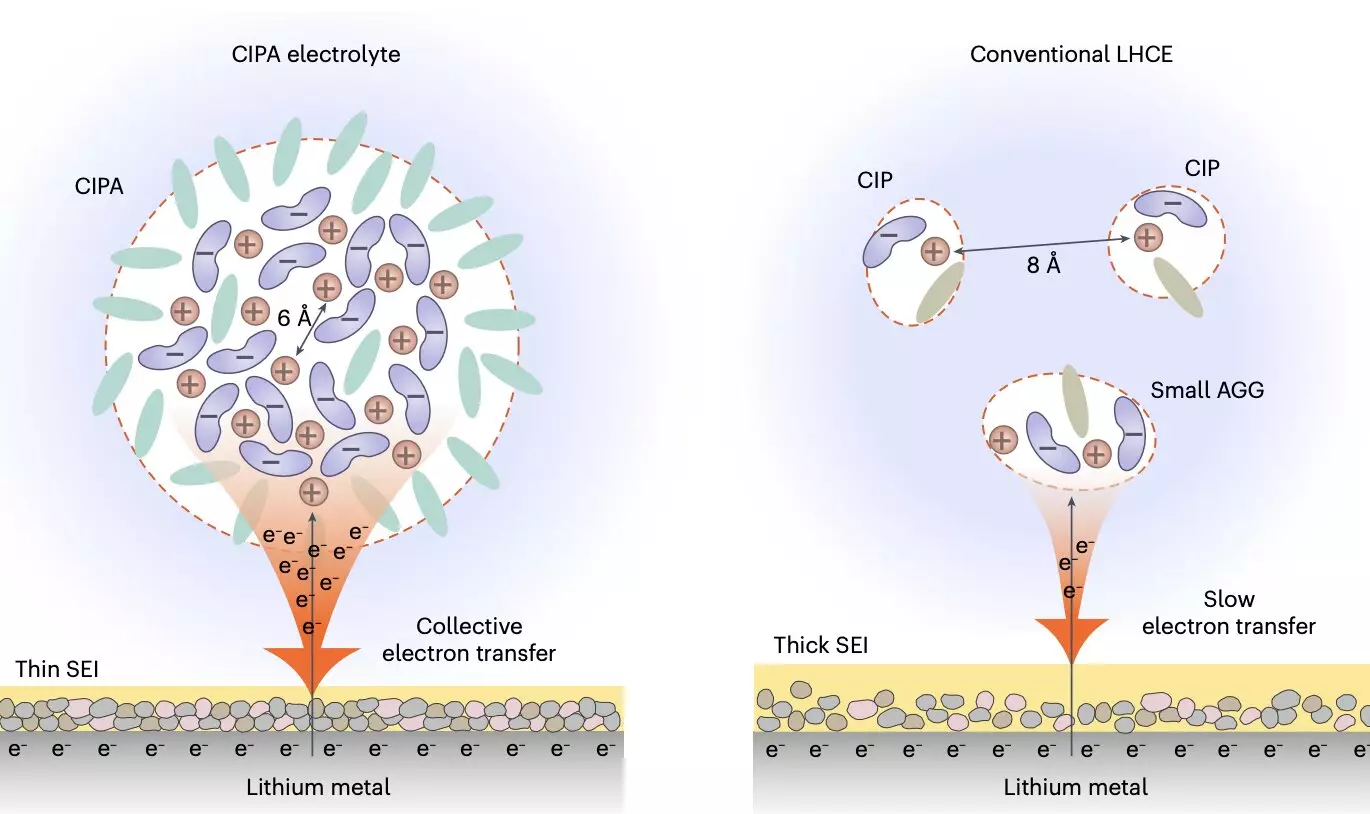
The recent study led by Prof. Jiao aimed to tune the solvation structure of the electrolyte at a mesoscopic level, focusing on the interaction between ion pairs that form the electrolyte’s aggregate structure. By creating compact ion-pair aggregates (CIPA) with closely packed lithium-anion ion pairs featuring coordination bonding, the researchers were able to achieve a stark improvement in the reduction of the lithium-metal anode. This unique design led to the rapid decomposition of anion clouds on the anode surface, forming a stable solid electrolyte interface (SEI) that effectively suppressed electrolyte decomposition and enabled homogeneous lithium deposition.
The newly designed electrolyte not only stabilized the lithium-metal anode but also exhibited good oxidative stability while suppressing the dissolution of transition metal elements from the cathode. This dual effect enhanced the stability of both the anode and cathode interfaces, ultimately leading to stable cycling for an extended number of cycles. The researchers’ innovative electrolyte design represents a significant breakthrough in the field of lithium-metal batteries, offering a new avenue for improving battery performance and lifespan.
The development of a novel electrolyte design by Prof. Jiao and her team opens up exciting possibilities for the future of lithium-metal batteries. By fine-tuning the solvation structure of the electrolyte, the researchers have achieved a significant improvement in battery performance and longevity. The successful creation of a 500 Wh/kg lithium-metal pouch cell retaining 91% of its energy after 130 cycles demonstrates the potential of this new electrolyte design. As researchers worldwide continue to explore and refine this technology, the future of lithium-metal batteries looks promising, with the potential for even higher energy densities and longer lifespans on the horizon.

Leave a Reply